溶胶凝胶法载银抗菌研究

Antimicrobial Effects and Human Gingival Biocompatibility of Hydroxyapatite Sol–Gel Coatings
Ren-Jei Chung,1Ming-Fa Hsieh,2,*Chine-Wen Huang,1Li-Hsiang Perng,3Hsiao-Wei Wen,4
Tsung-Shune Chin1
1Department of Materials Science and Engineering,National Tsing Hua University,101,Sec2,Kuang-Fu Road,Hsinchu 300,Taiwan,Republic of China
2Biomedical Engineering Center,Industrial Technology Research Institute,195Chung Hsing Road,Sec4Chu Tung, Hsinchu310,Taiwan,Republic of China
3Department of Chemical Engineering,Cheng Shiu University,840,Chengcing Road,Niaosong Township,Kaohsiung83305, Taiwan,Republic of China
4Nuclear Science and Technology Development Center,National Tsing Hua University,101,Sec2,Kuang-Fu Road,Hsinchu 300,Taiwan,Republic of China
Received7December2004;revised27March2005;accepted6April2005
Published online3August2005in Wiley InterScience(https://www.360docs.net/doc/0818527079.html,).DOI:10.1002/jbm.b.30365
Abstract:The sol–gel method was employed to synthesize hydroxyapatite(HAp)coatings
modi?ed with Ag or Zn ions onto Ti-6Al-4V substrate.A bacterial strain Streptococcus mutans
(S.mutans)and a human gingival?broblast(HGF-1)cell line were used to investigate the
antimicrobial effect and biocompatibility,respectively.HAp coatings containing100ppm Ag?
ions suppressed the growth of S.mutans.An apparent inhibition zone around the HAp coating
was further observed at Ag?concentration up to10,000ppm.However,for coatings contain-
ing Zn2?ions,a clear inhibition zone was observed at Zn2?concentration of10,000ppm.
Nevertheless,the results of HGF-1cultivation demonstrated that the Zn2?-modi?ed HAp
coatings exhibited better attachment and spread of HGF-1than did the Ag?-modi?ed coat-
ings.Zn2?modi?ed HAp coatings also increased the plating ef?ciency of HGF-1cells.The
cytotoxicity associated with the addition of Ag and the cell-conductive capacity associated with
the addition of Zn are proportional to the added concentration,from100to10,000ppm.The
dosages of both Ag?and Zn2?ions that should be added to HAp coatings were considered to
prevent infection and improve biocompatibility.The results of this study ensure that HAp
coatings modi?ed with a moderate amount of Ag/Zn ef?ciently resist microorganisms and
improve biocompatibility.?2005Wiley Periodicals,Inc.J Biomed Mater Res Part B:Appl Biomater76B:
169–178,2006
Keywords:hydroxyapatite;biocompatibility/hard tissue;Streptococcus mutans;human gin-
gival?broblast;antimicrobial;coatings
INTRODUCTION
Because they are corrosion-resistant,mechanically strong, tough,and reusable,titanium alloys have been applied in surgical implantations since the1940s.1,2Ti-6A1-4V is most commonly used in bony and dental restoration,especially in implant tooth roots.
Clinical studies have revealed that periimplant in?amma-tory diseases around dental implants may cause periimplan-titis and the loss of supporting bone.3,4Besides mechanical factors such as implant geometry and surface properties, bacterial contamination are also the major issue,leading to failure of dental implants.Pathogenesis of these diseases can be attributed to bacteria that normally reside in oral cavity. Some cariogenic bacterial strains,for example Streptococcus mutans(S.mutans),nourish dental implants in the presence of sucrose.5,6They produce extracellular glucan bio?lms on the surface of dental implants,resulting in accumulation of organic acid synthesized by the bacteria.Low pH in local environment persists over a long period will eventually lead to the erosion of hard tissues emerged as a result.It will
*Present address:Food Science and Technology Department,Cornell University,
Geneva,NY14456
Correspondence to:M.-F.Hsieh(e-mail:mfhsieh@https://www.360docs.net/doc/0818527079.html,.tw)
Contract grant sponsor:National Science Council of the Republic of China;contract
grant number:NSC91-2216-E-230-001
?2005Wiley Periodicals,Inc.
169
furthermore result in serious in?ammation and infection.Nevertheless,intra-oral translocation of other microorgan-isms makes healing process of periimplantitis much more complicated.Therefore,efforts on eliminating colonization of pathogenic species have been involved in the therapy of periimplant in?ammatory diseases and precautionary oral hygiene,3,4and for products preservation as well.
Hydroxyapatite [Ca 10(PO)6(OH)2,HAp]being the main inorganic component of hard tissues of vertebrates 7–9has been synthesized for various biomedical applications.10,11It has been coated on orthopedic and dental implants.In the previous studies,implants with HAp coating have many advantages:direct bony growth and early mineralization at the interface,providing greater ?xation strength in the early stage arisen from the ingrowth of bone tissue;restoration of injury through bony-induction ability of HAp;preventing acute foreign-body-effect caused by metal implant.There are various processes to coat HAp on titanium alloys,10such as plasma spraying,ion sputtering,chemical vapor deposition,electrochemical deposition,sol–gel route,and thermal de-composition,and so on.Among them,the sol–gel route is of relatively low temperature,the composition can be con-trolled,and it is suitable for large or irregular surface coat-ing.11
The ion exchange of HAp with metal ions is promising to create versatile functions of HAp in various applica-tions.12–14The present study is to synthesize metal-ion–added HAp coating and investigate its antimicrobial ef-fects against S.mutans .In the past decades,several metal ions have been used as antimicrobial elements,such as Ag ?,Zn 2?and Cu 2?,and so on 15,16in clinics.Among these,two metal ions were studied in this work:Ag ?and Zn 2?.The Ag-added or Zn-added HAp coatings were prepared via sol–gel method and then were investigated by X-ray diffraction for its crystalline phases.Moreover,it is required to further perceive whether this material still persists in good biocompatibility with peripheral gingival cells.In this regard,the cellular behavior of human gingi-val ?broblast on HAp coatings (pure and Ag/Zn added ones)on Ti-6A1-4V substrates was evaluated in vitro
.
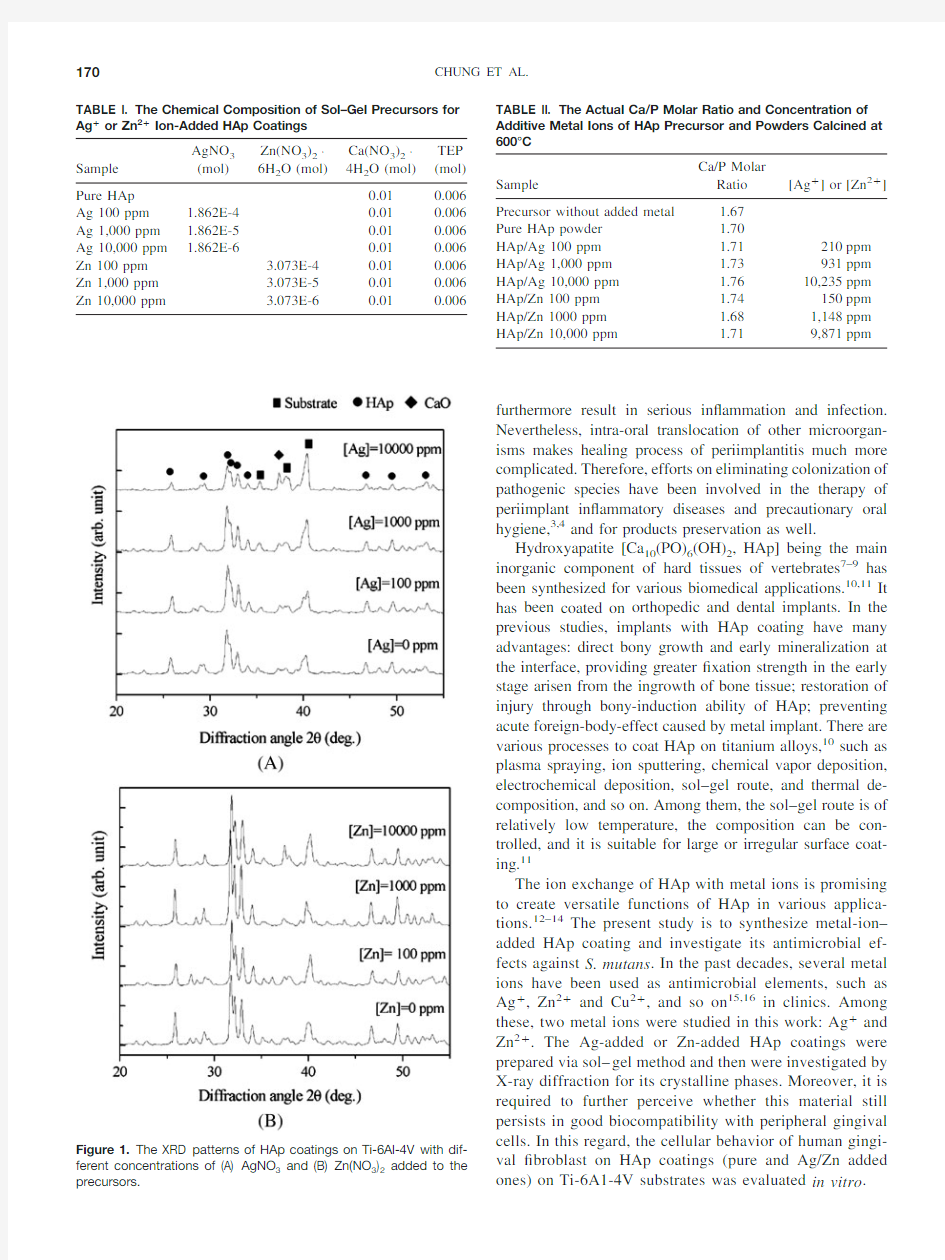
Figure 1.The XRD patterns of HAp coatings on Ti-6Al-4V with dif-ferent concentrations of (A)AgNO 3and (B)Zn(NO 3)2added to the precursors.
TABLE I.The Chemical Composition of Sol–Gel Precursors for Ag ?or Zn 2?Ion-Added HAp Coatings
Sample AgNO 3(mol)Zn(NO 3)2?6H 2O (mol)Ca(NO 3)2?4H 2O (mol)
TEP (mol)Pure HAp 0.010.006Ag 100ppm 1.862E-40.010.006Ag 1,000ppm 1.862E-50.010.006Ag 10,000ppm 1.862E-6
0.010.006Zn 100ppm 3.073E-40.010.006Zn 1,000ppm 3.073E-50.010.006Zn 10,000ppm
3.073E-6
0.01
0.006
TABLE II.The Actual Ca/P Molar Ratio and Concentration of Additive Metal Ions of HAp Precursor and Powders Calcined at 600°C
Sample
Ca/P Molar Ratio [Ag ?]or [Zn 2?]
Precursor without added metal 1.67Pure HAp powder 1.70HAp/Ag 100ppm 1.71210ppm HAp/Ag 1,000ppm 1.73931ppm HAp/Ag 10,000ppm 1.7610,235ppm HAp/Zn 100ppm 1.74150ppm HAp/Zn 1000ppm 1.681,148ppm HAp/Zn 10,000ppm
1.71
9,871ppm
170CHUNG ET AL.
MATERIALS AND METHODS
Preparation of Sol–Gel Precursors and HAp Coatings
The metal-ion–added HAp coatings,with precursor compo-sitions listed in Table I,were prepared by sol–gel technique,followed by a rapid thermal process.17Precursors with cal-cium to phosphorus molar ratio of 1.67were prepared by mixing Ca(NO 3)2-4H 2O,triethyl phosphate [(C 2H 5O)3PO,TEP)and AgNO 3(or/and Zn(NO 3)2]in 2-methoxy ethanol.The precursors were aged in closed containers at 80–90°C for 16h followed by drying in opened containers at the same temperature until its viscosity reached 100?10cP.The pH values were recorded throughout the aging and drying steps.The HAp gels were spin-coated on Ti-6Al-4V alloy sub-strates that had been surface blasted by 500–850-?m Al 2O 3beads,and then predried at 150°C,followed by a rapid heating to 400°C (at a ramp 300°C/min)in ambient environ-ment and holding for 3min.The coatings were repeatedly deposited four more times,and the ?nal thick coatings were subjected to a rapid heating to 600°C (at a ramp 300°C/min)and holding for 1min.
Chemical and Physical Characterizations of the HAp
Powders and Coatings
Due to insuf?cient quantity of the coatings for detailed chem-ical characterization,powders with the same stoichiometry as the coatings were also prepared.The preparation procedure was to calcine the said gels following the same heating process.The concentrations of silver or zinc and Ca/P molar ratio in the calcined HAp powders were analyzed by an inductively coupled plasma mass spectrometer (Perkin Elmer,SCIEX ELAN 5000).The precursors receiving aging and drying steps (mentioned in the last paragraph)were calcined at 500°C in alumina crucibles.Twenty-?ve micro-grams of as-calcined powders were digested by 3mL HNO 3(70%)and 0.5mL H 2O 2in microwave oven (Milestone Co.,mls 1200)for 2min.The resultants were then analyzed.The HAp coatings were examined by an X-ray diffractom-eter (Geiger ?ex D/MAX II B,Rigaku Co.)with the use of Cu K ?radiation.The JCPDS (Joint Committee on Powder Dif-fraction Standards)for HAp (Card No.09-0432)and CaO (Card No.37-1497)were used to identify phase composi-tions.18The surface morphology of HAp coatings was
ob-
Figure 2.FE-SEM morphology of HAp coatings added with different concentrations of (A)pure HAp,(B)100ppm Ag,(C)1,000ppm Ag,(D)10,000ppm Ag,(E)1,000ppm Zn,and (F)10,000ppm Zn.
171
HYDROXYAPATITE SOL–GEL COATINGS
served by a ?eld emission scanning electron microscope (FE-SEM,model JSM-6500F,JEOL,Japan).
In Vitro Antimicrobial Tests and the Cell Responses to
the HAp Coatings
To avoid biological contamination during in vitro studies,all samples were sterilized by gamma-ray irradiation with a total dosage of 20kGy before experiments.S.mutans (ATCC 25175)was cultured at 37°C in the brain heart infusion medium (BHI,Di?co.Co.)and brain heart infusion agar (BHIA,Di?co Co.)for cell propagation and antimicrobial tests,respectively.The antimicrobial tests of HAp coatings were conducted in BHIA solid media with the spread-plate method.0.1mL of S.mutans cell suspension (1.5?104CFU/mL)was spread on a 12-cm BHIA plate,and a sample of the HAp coating side was put on it for a time period of 48h.
Human gingival ?broblast (HGF-1,ATCC CRL-2014)from passages numbered 6–20was cultured in Dulbecco’s modi?ed eagle medium (DMEM)supplemented with heat-inactivated fetal bovine serum (10%v/v)in an incubator at
37°C,with 5%CO 2and a saturated humidi?ed atmosphere.0.1mL of HGF-1cell suspension (105cells/mL)was seeded directly onto the HAp surface of the Ti-6Al-4V substrates in a 24-well dish,and then incubated for 65h.Triplicates were used under each condition.
A stereo optical microscope (SMZ-U,Nikon),supple-mented with re?ective light with a ?ber-optic light source (Nikon)and FE-SEM were used to observe the morphologies of the HAp/Ti-6Al-4V samples.The HGF-1cells were then stained with crystal violet and observed under a stereo optical microscope.The sample for FE-SEM observation was pre-pared as a separate HAp/Ti-6Al-4V sample seeded with HGF-1cells treated with 3mL solution of 2.5%glutaralde-hyde for 1h at room temperature.Then the samples were gently washed twice with 3mL of phosphate buffered saline (PBS)to remove any suspended cells,and dehydrated through a graded series of alcohol solutions (40–100%),each for 15min.The samples underwent critical point drying (CPD)and were coated with platinum for FE-SEM examina-tion.
The acid phosphatase (ACP)assay was used to measure the population of HGF-1cells.19The ACP assay is sensitive and suitable for the evaluation of small cell population.The mechanism is used to determine the activity of the cytosolic acid phosphatase with the use of a synthetic substrate of p -nitrophenylphosphate (P NPP).PNPP reacted with ACP followed by formation of p -nitrophenol.The concentration of p -nitrophenol can be measured by UV-VIS spectrometer.So the concentration of ACP in the HGF-1cells can be quanti-tatively determined.After plating and culturing the cells on the coatings for 48h,a 500-?L aliquot of buffer,containing 0.1M sodium acetate (pH 5.5,J.T.Baker Chemical,Phil-lipsburg,NJ),0.1%(v/v)Triton X-100(BDH Chemicals)and 10mM p -nitrophenyl phosphate (Sigma 104phosphatase substrate,Sigma Chemical),was added to each culture well,and the culture wells were kept in the incubator at 37°C for 2h.The reaction was stopped by the addition of 50?L 1M NaOH to each well,and the wells were measured at a wave-length of 405nm with the use of an enzyme-linked immu-nosorbent assay reader.
RESULTS AND DISCUSSION Material Characterization
Prepared Sol–Gel Precursors.Sol–gel derived HAp
ceramic is sensitive to the aging process,especially when organic phosphate-or phosphite-based precursors were used.17,20The sol solutions under aging were transparent in spite of silver or zinc additions,and pH values de-creased from 3.0–4.0to 2.0in a period of 16h.Note that the sol solutions became pale yellow at the end of aging.Theoretically,the chemistry of the aging process is com-plicated because of two concurrent reactions:the hydroly-sis of phosphate and the formation of Ca-P intermedi-ates.20Thus metal ions,Ag ?or Zn 2?dissolved in the precursors may affect Ca-P intermediates because of
sub-
Figure 2.(continued)
172CHUNG ET AL.
stitutions by Ag ?or Zn 2?in the Ca-P intermediates.However,no differences in color and pH values of sol solutions were seen in the aging process,and the sol remained transparent without any precipitate.
The effect of Ag ?or Zn 2?addition appeared signi?cant in the drying and calcination.The phase evolved after precur-sors were spin-coated onto Ti-6Al-4V substrates and rapidly calcinated,and showed HAp main phase along with an im-pure phase CaO.Figure 1displays XRD patterns of HAp coating.The CaO impurity was enhanced with increasing metal addition,which revealed a positive substitution trend of the additive metal ions.21It was inferred that the substitution of silver or zinc ions in Ca-P intermediates of aged sol resulted in more free calcium ions.Thus,upon rapid heating of HAp precursors coated on the substrates,calcium ions reacted with surrounding oxygen,leading to CaO by-prod-ucts.Yet the effect of substrate material was also suspected as one of the reasons.HAp deposited over silicon substrates also contained CaO phase,regardless of silver or zinc addition.Lopatin et https://www.360docs.net/doc/0818527079.html,ed silicon wafers coated with borophosphate silicate as substrates and reported CaO formation as a minor phase.22
Chemical Compositions of HAp Powder.Table II sum-
marizes the Ca/P molar ratio of HAp powders prepared at 600°C.The ratios were in the range of 1.67(precursors)to 1.76(10,000ppm silver added).The higher Ca/P ratios,except precursors,can be anticipated as organic phosphorus compounds are more susceptible to evaporate upon calcining in an open-air oven as compared with calcium nitrate.On the other hand,in previous reports,species of carbonated HAp [for instance,Ca 10(PO 4)6-X (CO 3)3/2X (OH)2]are commonly observed in arti?cial and bioinspired apatites.21The substi-tute carbonate groups would lead to raising of Ca/P ratio in a reasonable manner.Phase purity represented in Figure 1ensures the ef?cacious production of crystalline HAp.
Table II also shows the measured concentration of additive metal ions.The actual silver concentration in sample Ag 100ppm was 210ppm,which is two times more than the desig-nated amount in the precursors (Table I,Ag 100ppm).In the HAp powders using the precursors of Zn 1,000ppm and Zn 10,000ppm,the actual zinc concentrations were 1148ppm and 9871ppm,respectively.The silver and zinc concentra-tions in the HAp powders seem not to match with designated precursors exactly;however,the measured data are within
a
Figure 3.Antimicrobial tests of HAp coatings on BHIA (with a ruler in millimeters):(A)pure HAp coatings;(B)HAp coatings with 100ppm Ag added (arrow indicated the effect due to poor spreading of precursor);(C)HAp coatings with 10,000ppm Ag added;(D)HAp coatings with 1,000ppm Zn added;(E)HAp coatings with 10,000ppm Zn added;(F)PSA coated on blasted Ti-6Al-4V substrate (samples a and c)and pure HAp coatings (samples b and d).Samples c and d were washed by phosphate buffer and distilled water three times.[Color ?gure can be viewed in the online issue,which is available at https://www.360docs.net/doc/0818527079.html,.]
173
HYDROXYAPATITE SOL–GEL COATINGS
reasonable error range,and most importantly the increasing trend persists.There is no evidence of phase separation for the additives.Synthesized coatings are homogeneous and with distinguishable characteristics,as in the original design.
HAp Coating.The total thickness of the coating was
around 20?m for ?ve repeated spinning coats.The FE-SEM micrograph revealed that pure HAp had a rough and porous surface with pore sizes ranging from 1to 10?m [Figure 2(A)].The porous structure is a characteristic feature of rapid thermal process.The micrographs also showed that,with increasing added concentration of Ag,the surface of HAp coatings was more compact [Figure 2(B–D)].On the other hand,increasing Zn addition leads to ?ner microstructure with cellulose morphology [Figure 2(E–F)].
Compared to plasma spraying and the biomimetic method,10,23the sol–gel route for HAp coating is an econom-ical and time-saving process.It is also suitable for coating complex surfaces.Another advantage is that the incorpora-tion reagents are able to homogeneously distribute without phase separation.The coating thickness can be adjusted through precursor viscosity and sequential coatings.Regard-ing about the antibiotic carriers,the reagent is also distributed deep in the coating;a steady and long-period releasing effect is anticipated.
In Vitro Antimicrobial Tests of HAp Coatings
S.mutans was used as the tested bacterial strain.The interactions between S.mutans and HAp coating modi?ed with metal ions were observed.S.mutans ,a Gram-positive bacteria,is an etio-logical agent of human caries and plaques,and has been exten-sively investigated in the pathogenesis of oral infection.A mi-croassay conducted for bacterial adherence to HAp revealed that S.mutans attaches onto the HAp surface by glucan-mediated accretion.24Calcium-phosphate coatings on dental implants are usually loaded with antibiotics to prevent postoperative bacterial infectionly.25However,the prevalence of bacterial antibiotic resistance has become a serious issue in medical communities around the world;this study seeks to develop HAp coatings to which silver or zinc has been added,aiming at tackling the issues of antibiotic resistance.
In the antimicrobial tests,the growth of S.mutans around the coating layers of pure HAp were observed,the microbial colonies were uniformly distributed [Figure 3(A)].However,the Ag 100ppm coating suppressed the growth of the S.mutans that surrounded the sample [Figure 3(B)].Some bac-terial colonies remained close to the corners of the coating [indicated by an arrow in Figure 3(B),because of the poor spreading of the precursor during the spin-coating process.Furthermore,the HAp ?lm added with 10,000ppm Ag com-pletely inhibited the growth [Figure 3(C)].Adding 1,000ppm Zn to the HAp coatings [Figure 3(D)]did not clearly suppress the growth,but adding 10,000ppm Zn did [Figure 3(E)].The use of antibiotics as a substitute for metal ions and the incorporation into the porous HAp coatings that serve as carriers were also evaluated.An antibiotic complex of peni-cillin (10,000units/mL),streptomycin (10mg/mL),and am-photericin (0.025mg/mL)(PSA)was absorbed directly onto Ti-6Al-4V substrate [Figure 3(F),samples a and c]or on pure HAp coatings [Figure 3(F),samples b and d].Samples c and d in Figure 3(F)were washed with the phosphate buffer and distilled water three times each.Neither the coated [Figure 3(F),sample d]nor the noncoated [Figure 3(F),sample c]batch exhibited a stronger antimicrobial effect.Figure 3(F),samples a and b show obvious antimicrobial phenomenon.The antibiotic complex immobilized by the substrate surface and the coating effectively inhibited the proliferation of bac-teria.However,after they had been washed,only coated samples exhibited slight antimicrobial ef?cacy [Figure 3(F),sample d];treated naked Ti-6Al-4V substrate exhibited no antimicrobial ability,unlike in Figure 3(F),sample a.It is suggested that the surface of HAp coatings did not strongly trap PSA antibiotics,which were easily removed by ?uid.Stiger,Groot,and Layrolle conducted a similar investigation by absorbing tobramycin into biomimetic HAp coatings or plasma-sprayed HAp coatings.They found that 90%of the antimicrobial agent was released within 3h.25Radin et al.and Campbell et https://www.360docs.net/doc/0818527079.html,ed multilayer coatings as barriers to retard the quick release of antibiotics and thereby cause long-term release.However,the outer coating also inhibited mammal cell adhesion,even though the release period was extended to as long as 72h.
26,27
Figure 3.(continued)[Color ?gure can be viewed in the online issue,which is available at https://www.360docs.net/doc/0818527079.html,.]
174CHUNG ET AL.
The prevention and treatment of postsurgical infection remain challenging tasks in orthopedics and dentistry.Infec-tion results in early failure.Sterilization and antiinfection procedures are necessary not only during implantation sur-gery,but also after changing dressings.Hence,a long-term antimicrobial strategy is crucial.The antimicrobial effect of organic antibiotics carried by HAp coatings is manifest pri-marily through physical absorption;thereafter,rapid dissolu-tion is unavoidable.HAp to which Ag or Zn has been added,consolidated by rapid thermal process,features slow release,and the tissue is directly attachable to the bony inductive materials.Restated,adding a moderate or an appropriate speci?c amount of metallic ions to an HAp coating offers advantages of long-term microbial suppression beyond just short-range
effects.
Figure 4.Morphology of HGF-1cells cultured on a ?ask observed through a stereo optical microscope.[Color ?gure can be viewed in the online issue,which is available at
https://www.360docs.net/doc/0818527079.html,.]
Figure 5.Morphology of HGF-1cells attached on different HAp coatings observed through a stereo optical microscope after crystal violet staining (original magni?cation 200?;arrows indicate the cells;the scale bar is 100?m).(A)Pure HAp coatings;(B)1,000ppm Ag added;(C)10,000ppm Ag added;(D)1,000ppm Zn added;(E)10,000ppm Zn added.[Color ?gure can be viewed in the online issue,which is available at https://www.360docs.net/doc/0818527079.html,.]
175
HYDROXYAPATITE SOL–GEL COATINGS
Attachment and Plating Ef?ciency Of Human Gingival
Fibroblast to HAp Coatings
Figure 4shows the HGF-1cell morphology observed under a stereo optical microscope on a culture ?ask.The cells had a spindle shape;the cell bodies were 5?m wide and 60–70?m long,and their pseudopods were extended from two distal ends.In the literature,primary gingival ?broblasts extracted from adult humans and plated on calcium-phosphate surfaces exhibited doubling times of a few days to 1week.28The HGF-1cells cultured in the T75?asks were slowly expended;the estimated doubling time was about 2weeks.In this study,the HGF-1cell culture was used to investigate the short-term attachment of cells over HAp coatings.Figure 5presents the morphology of HGF-1cells cultured on HAp coatings for 48h.On the pure HAp coating [Figure 5(A)],attached cells (indicated by arrows)were observed with extended pseudo-pods,revealing that the pure HAp surface was not cytotoxic to the cells.However,cells seeded on HAp coatings to which Ag had been added [Figure 5(B,C),1,000and 10,000ppm respectively)did not spread very well;those on coatings to which Zn had been added [Figure 5(D,E),1,000and 10,000
ppm,respectively]extended their pseudopods and compactly adhered onto the HAp coating surface.Samples were then examined by FE-SEM following CPD.The surface
morphol-
Figure 6.Morphology of HGF-1cells attached on different HAp coatings observed through FE-SEM (original magni?cation 1000?):(A)pure HAp coatings;(B)1,000ppm Ag added;(C)1,000ppm Zn added;(D)10,000ppm Zn
added.
Figure 7.Number of attached HGF-1cells onto different HAp coat-ings evaluated by ACP assay.[Color ?gure can be viewed in the online issue,which is available at https://www.360docs.net/doc/0818527079.html,.]
176CHUNG ET AL.
ogy of the coating changed because of etching by immersion in the medium and CPD processing,providing clearer details of the adherence(Figure6).The pseudopods of the attached cells were in direct contact with the coatings,and the cells presented extended morphologies.Cells on HAp coatings to which Zn had been added seemed to be active.
Figure7plots the results of ACP assay for various HAp coatings after they were cultured for65h.The optimized plating ef?ciency onto the culture dish was30%.When the concentration of added Ag was increased(100to10,000 ppm),the cytotoxicity of the corresponding coating in-creased.Subsequently,fewer cells adhered and subsisted onto the surface.However,as more Zn was added(100to10,000 ppm),more cells survived.Adding Zn to the HAp coating not only promoted the early attachment of the HGF-1cells,but also increased the plating ef?ciency.In vitro evaluation di-rectly revealed the immediate response of the cells to the coating.The in situ environment of implantation is more complex,and animal tests are being conducted to ensure the developed coatings can be used.
Results of the in vitro HGF-1tests reveal that the cells directly adhere to the HAp coatings with added Ag/Zn.The cytotoxicity of Ag-HAp coatings depends on the Ag concen-tration;increasing the amount of added Zn increases the biocompatibility of Zn-HAp.Table III presents the results of antimicrobial and in vitro biocompatibility tests.A combina-tion of Ag and Zn is preferred,and the optimal mixing concentration is currently under investigation.A HAp coating with added Ag and Zn exhibits effective antimicrobial ability and satisfactory biocompatibility.An effort should be made to design an appropriate surface for antibiotic entrapment and organic–inorganic hybrid surface entrapment with Ag/Zn to reduce the cost of dental implants.
CONCLUSIONS
The sol–gel method is a suitable process for preparing Ag?-or Zn2?-added antimicrobial HAp coating,enabling the infection prevention of implantation.In order to achieve early-stage healing and prevent postsurgical in?ammation,a biocompatible and antimicrobial HAp coating on metal im-plant is needed.In this study,adding100ppm Ag ions to the sol–gel precursor calcined HAp coatings on Ti-6Al-4V sub-strates resulted in growth suppression of the pathogen(Strep-tococcus mutans),and an apparent inhibition zone was found for HAp?lm with Ag ion addition up to10,000ppm.For Zn-added HAp,more than1,000ppm Zn is needed to show antimicrobial effect.Zn addition(100–10,000ppm)is bene-?cial for cell attachment and intends to increase the plating ef?ciency of human gingival?broblast cells.
REFERENCES
1.Bothe TR,Beaton LE,Davenport HA.Reaction of bone to
multiple metallic implants.Surg Gynecol Obstet1940;71:598–602.
2.Meffert RM,Langer B,Fritz ME.Dental implants—A review.
J Periodontol1992;63:859–870.
3.Quirynen M,De Soete M,van Steenberghe D.Infectious risks
for oral implants:A review of the literature.Clin Oral Implants Res2002;13:1–19.
4.Grivet M,Morrier JJ,Benay G,Barsotti O.Effect of hydropho-
bicity on in vitro streptococcal adhesion to dental alloys.J Mater Sci Mater Med2000;11:637–642.
5.Bates DG,Navia JM.Chemotherapeutic effect of zinc on Strep-
tococcus mutans and rat dental-caries.Arch Oral Biol1979;24: 799–805.
6.Liljemark WF,Bloomquist C.Human oral microbial ecology
and dental caries and periodontal diseases.Crit Rev Oral Biol Med1996;7:180–198.
7.Jarcho M.Calcium phosphate ceramics as hard tissue prosthet-
ics.Clin Orthop1981;157:259–278.
8.Hench LL.Bioceramics—from concept to clinic.J Am Ceram
Soc1991;74:1487–1510.
9.de Groot K,Wolke JGC,Jansen JA.Calcium phosphate coat-
ings for medical implants.Proc Inst Mech Eng H1998;212: 137–147.
10.Ong JL,Chan DCN.Hydroxyapatites and their use as coatings
in dental implants:A review.Crit Rev Biomed Eng2000;28: 667A–707A.
11.Gross KA,Chai CS,Kannangara GSK,Ben-Nissan B,Hanley
L.Thin hydroxyapatite coatings via sol–gel synthesis.J Mater Sci Mater Med1998;9:839–843.
12.Root MJ.Inhibition of the amorphous calcium phosphate trans-
formation reaction by polyphosphonates and metal ions.Calcif Tissue Int1990;47:112–116.
13.Hidaka S,Okamoto Y,Abe K.Elutions of metal-ions from
dental casting alloys and their effect on calcium-phosphate precipitation and transformation.J Biomed Mater Res1994;28: 175–180.
14.Bigi A,Foresti E,Gandol?M,Gazzano M,Rovri N.Inhibiting
effect of zinc on hydroxyapatite crystallization.J Inorg Bio-chem1995;58:49–58.
15.Okamoto Y,Hidaka S.Studies on calcium phosphate precipi-
tation:Effects of metal ions used in dental materials.J Biomed Mater Res1994;28:1403–1410.
16.Kim TN,Feng QL,Kim JO,Wu J,Wang H,Chen GC,Cui FZ.
Antimicrobial effects of metal ions(Ag?,Cu2?,Zn2?)in hydroxyapatite.J Mater Sci Mater Med1998;9:129–134. 17.Hsieh MF,Perng LH,Chin TS,Perng HG.Phase purity of
sol–gel-derived hydroxyapatite ceramic.Biomaterials2001;22: 2601–2607.
18.JCPDS.Joint Committee on Powder Diffraction Standards
(JCPDS)database of standards:Powder diffraction(inorganic and
TABLE III.In Vitro Antimicrobial and Biocompatibility Tests of Ag/Zn Substituted HAp Coatings(Ranging from?to???) Sample Antimicrobial Zone a HGF-1Cell Attachment b Pure HAp??
Ag100ppm???
Ag1,000ppm????
Ag10,000ppm????
Zn100ppm??
Zn1,000ppm??
Zn10,000ppm??????
a Distance of the edge of apparent antimicrobial zone to the coatings(?,2mm;??, 2–5mm;???,?5mm).
b Attached cell number evaluated by ACP assays(?,0–1300cells;??,1300–2600 cells;???,2600–4000cells).177
HYDROXYAPATITE SOL–GEL COATINGS
organic).Swarthmore,PA:International Centre for Diffraction Data,and American Society for Testing and Materials;2003. 19.Roach HI.New aspects endochondral ossi?cation in the chick:
chondrocyte apoptosis,bone formation by former chondrocytes, and acid phosphatase activity in the endochondral bone matrix.
J Bone Miner Res1997;12:795–805.
20.Liu DM,Troczynski T,Tseng WJ.Aging effect on the phase
evolution of water-based sol–gel hydroxyapatite.Biomaterials 2002;23:1227–1236.
21.Chung RJ,Hsieh MF,Huang KC,Perng LH,Chou FI,Chun TS.
Anti-microbial hydroxyapatite particles synthesized by a sol–gel route.J Sol–Gel Sci Technol.Forthcoming.
22.Lopatin CM,Pizziconi V,Alford TL,Laursen T.Hydroxyapa-
tite powders and thin?lms prepared by a sol–gel technique.
Thin Solid Films1998;326:227–232.
23.Habibovic P,Barrere F,van Blitterswijk CA,de Groot K,
Layrolle P.Biomimetic hydroxyapatite coating on metal im-plants.J Am Ceram Soc2002;85:517–522.24.Schilling KM,Carson RG,Bosko CA,Golikeri GD,Bruinooge
A,Hoyberg K,Waller AM,Hughes NP.A microassay for bacterial adherence to hydroxyapatite.Colloid Surf B1994;3: 31–38.
25.Stiger M,Groot KD,Layrolle P.Incorporation of tobramycin
into biomimetic hydroxyapatite coating on titanium.Biomate-rials2002;23:4143–4153.
26.Radin S,Campbell JT,Ducheyne P,Cuckler JM.Calcium
phosphate ceramic coatings as carriers of vancomycin.Bioma-terials1997;18:777–782.
27.Campbell AA,Song L,Li XS,Nelson BJ,Bottoni C,Brooks
DE,Dejong ES.Development,characterization,and anti-micro-bial ef?cacy of hydroxyapatite-chlorhexidine coatings produced by surface-induced mineralization.J Bone Miner Res2000;53: 400–407.
28.Chou L,Marek B,Wagner WR.Effects of hydroxyapatite
coating crystallinity on biosolubility,cell attachment ef?ciency and proliferation in vitro.Biomaterials1999;20:977–985.
178CHUNG ET AL.
氧化锌制备方法
将mol·L-1的NaOH乙醇溶液缓慢滴加到含有mol·L-1的Zn(NO3)2·6H2O乙醇溶液中. 将混合溶液转移至高压反应釜中, 在130℃下反应12 h, 将反应产物经二次去离子水、乙醇等洗涤后, 在130 摄氏度下干燥,即可获得纯ZnO纳米棒. 在 ZnCl2 溶液 mol/L) 中加入一定量的 SDS, 搅拌下于 65 ℃将 Na2CO3 溶 液滴加到该溶液中 (120 滴/min, n(Na 2CO 3 )/n(ZnCl2) = 2),恒温反应 h. 将反 应液倒入聚四氟乙烯罐中, 在150~160 ℃进行水热反应 12 h, 自然冷却后离心分离, 用去离子水洗涤到无水Cl?离子, 再用无水乙醇洗涤 2~3 次, 50 ℃真空干燥 2 h, 300 ℃焙烧 3 h, 即制得 ZnO 纳米管. 将0. 1 L0. 1 mo l/ L二水合醋酸锌的乙醇溶液置于带冷凝管和干燥管的0. 5 L 圆底烧瓶中, 在80 ℃搅拌3 h, 不断收集冷凝物, 最后可获得0. 04 L 中间物和0. 06 L 冷凝物. 将中间物迅速用冷的绝对乙醇稀释至0. 1 L, 冷至室温, 得0. 1 mol/ L 中间产物. 氨水沉淀法制备纳米氧化锌 在水——乙醇介质中用氨水沉淀法制备出了纳米Zn(OH) 2 和ZnO材料,讨论了介质组成对沉淀产物ZnO微粒的粒径范围及形貌的影响,并研究出由Zn(OH)2分解为纳米ZnO的最佳干燥脱水条件为200℃、2h。表明本方法不需高温处理就可得到颗粒均匀且分布窄的ZnO纳米材料,粒径可达17~6nm。 一、试剂与仪器 主要原料为氯化锌、无水乙醇、氨水等,均为分析纯试剂。 仪器为微型滴定管、磁力搅拌器、恒温干燥烘箱。 二、试验方法 以水——乙醇为溶剂,其中醇的体积含量分别为0%(去离子水)、20%、60%、100%。将氯化锌、氨水配制成不同浓度的溶液(不同浓度是多少)。取一定体积(一定体积是多少)的氯化锌乙醇溶液于烧杯中,加以适当速度搅拌,不同浓度的氨水从微型滴管中缓慢滴入氯化锌乙醇溶液中,使之进行反应。控制氨水用量,调节pH值为左右,确定滴定终点。反应得到的白色沉淀物,经抽滤洗涤后自然风干 即为Zn(OH) 2纳米粉,Zn(OH) 2 经干燥(200℃、2h)脱水后,为ZnO纳米粉
溶胶凝胶法制备材料
溶胶-凝胶法制备材料 摘 要:溶胶-凝胶法广泛应用于制备薄膜材料和粉体材料,其主要原理是将金属醇盐或无机盐经水解直接形成溶胶或经解凝形成溶胶,然后使溶质聚合凝胶化,再将凝胶干燥、焙烧去除有机成分,最后得到无机材料。本文主要介绍了一些溶胶-凝胶法制备材料的发展历史,原理以及一些溶胶-凝胶法实际应用案例。 关键词:溶胶-凝胶法;纳米材料;陶瓷薄膜材料;掺杂;锂电池;包覆材料 溶胶-凝胶法发展过程:1846年法国化学家J.J.Ebelmen 用SiCl 4与乙醇混合后,发现在湿空气中发生水解并形成了凝胶。20世纪30年代W.Geffcken 证实用金属醇盐的水解和凝胶化可以制备氧化物薄膜。1971年德国H.Dislich 报道了通过金属醇盐水解制备了SiO 2-B 2O-Al 2O 3-Na 2O-K 2O 多组分玻璃。1975年 B.E.Yoldas 和M.Yamane 制得整块陶瓷材料及多孔透明氧化铝薄膜。80年代以来,在玻璃、氧化物涂层、功能陶瓷粉料以及传统方法难以制得的复合氧化物材料得到成功应用。 分类:溶胶-凝胶法按产生溶胶凝胶过程机制主要分成三种类型: (1)传统胶体型:通过控制溶液中金属离子的沉淀过程,使形成的颗粒不团聚成大颗粒而沉淀得到稳定均匀的溶胶,再经过蒸发得到凝胶。 (2)无机聚合物型:通过可溶性聚合物在水中或有机相中的溶胶过程,使金属离子均匀分散到其凝胶中。常用的聚合物有聚乙烯醇、硬脂酸等。(3)络合物型:通过络合剂将金属离子形成络合物,再经过溶胶,凝胶过程成络合物凝胶。 制备方法及原理:溶胶一凝胶科学技术是以金属醇盐为原料制作玻璃、玻璃陶瓷、陶瓷以及其它功能无机材料的一种新工艺方法。溶胶-凝胶法制备材料的方法属于化学制备方法,溶胶-凝胶体的制备有3种途径:(1)溶胶溶液的凝胶化; (2)醇盐或硝酸盐前驱体的水解聚合,继之超临界干燥凝胶;(3)醇盐前驱体的水解聚合。 溶胶-凝胶法的化学过程首先是将原料分散在溶剂中,然后经水解反应生成活性单体,活性单体进行聚合,开始成为溶胶,进而生成具有一定空间结构的凝胶,经过干燥和热处理制备出纳米粒子和所需材料。其基本反应式为: ;)()()(424nHOR OH OR M O nH OR M n n +→+-水解: ;])()([)(22214-4O H O OH OR M OH OR M n n n n +→--)(缩聚:
溶胶凝胶法
溶胶凝胶法 定义:采用合适的有机或无机盐配制成溶液,然后加入能使之成核、凝胶化的溶液, 控制其凝胶化过程得到具有球形颗粒的凝胶体,经一定温度煅烧分解得到所需物相的 方法。 应用学科:百科名片 溶胶-凝胶法就是用含高化学活性组分的化合物(无机物或金属醇盐)作前驱体,在液相 下将这些原料均匀混合,并进行水解、缩合化学反应,在溶液中形成稳定的透明溶胶体系,溶胶经陈化胶粒间缓慢聚合,形成三维空间网络结构的凝胶,凝胶网络间充满了失去流动 性的溶剂,形成凝胶。凝胶经过干燥、烧结固化制备出分子乃至纳米亚结构的材料。 优点 溶胶-凝胶法与其它方法相比具有许多独特的优点:(1)由于溶胶-凝胶法 中所用的原料首先被分散到溶剂中而形成低粘度的溶液,因此,就可以在很短 的时间内获得分子水平的均匀性,在形成凝胶时,反应物之间很可能是在分子 水平上被均匀地混合。(2)由于经过溶液反应步骤,那么就很容易均匀定量 地掺入一些微量元素,实现分子水平上的均匀掺杂。(3)与固相反应相比, 化学反应将容易进行,而且仅需要较低的合成温度,一般认为溶胶一凝胶体系 中组分的扩散在纳米范围内,而固相反应时组分扩散是在微米范围内,因此反 应容易进行,温度较低。(4)选择合适的条件可以制备各种新型材料。溶胶一凝胶法也存在某些问题:通常整个溶胶-凝胶过程所需时间较长(主要指陈化时间),常需要几天或者几周;还有就是凝胶中存在大量微孔,在干燥过程 中又将会逸出许多气体及有机物,并产生收缩 发展历史 1846年法国化学家J.J.Ebelmen用SiCl4与乙醇混合后,发现在湿空气中发生 水解并形成了凝胶。 20世纪30年代W.Geffcken证实用金属醇盐的水解和凝胶化可以制备氧化物薄膜。 1971年德国H.Dislich报道了通过金属醇盐水解制备了SiO2-B2O-Al2O3-Na2O-K2O多组分玻璃。 1975年B.E.Yoldas和M.Yamane制得整块陶瓷材料及多孔透明氧化铝薄膜。
ZnO合成方法
存档日期:存档编号: 北京化工大学 研究生课程论文 课程名称:纳米材料化学 课程代号:ACh530 任课教师:左胜利 完成日期:2011 年12 月8 日 专业:化学 学号:2011200989 姓名:李浩 成绩:
ZnO纳米材料的制备与应用 摘要 本篇综述从制备方法和应用领域出发,论述了制备ZnO纳米材料的一些常用方法如直接沉淀法、微乳液法、溶胶-凝胶法、模板法、水热合成法等,并简单介绍了氧化锌纳米材料在环境、食品、油漆涂料、橡胶、塑料、树脂、纺织品、化妆品等领域的应用。 关键词:ZnO纳米材料制备应用
目录 前言 (1) 第1章氧化锌纳米材料的结构与性质 (2) 1.1节氧化锌纳米材料的结构 (2) 1.2节氧化锌纳米材料的主要性质 (2) 第2章氧化锌纳米材料的制备方法及应用领域 (4) 2.1节氧化锌纳米材料的制备方法 (4) 2.2节氧化锌纳米材料的主要应用领域 (6) 结论 (8) 参考文献 (9)
前言 19世纪末到20世纪初,人类对微观世界的认识已经延伸到一定层次,时间上已经达到了纳秒、皮秒和微妙的数量级。随着研究的深入,20世纪70年代,人类开启了规模生产纳米材料的历史。纳米微粒狭义上是指有关原子团簇、纳米颗粒、纳米线、纳米薄膜、纳米碳管、纳米固体材料的总称,而广义上则指晶粒或晶界等显微构造能达到纳米尺寸材料。该新型材料必将以其独特的量子尺寸效应、小尺寸效应、表面效应及宏观量子隧道效应等性质在各个领域崭露头角。例如复合材料、大规模集成电路、超导线材料多相催化等方面的开发及应用。 近年来,纳米材料的合成方法及应用领域受到了研究者的广泛关注,TiO2、ZnO、CaF2、Al2O3纳米材料的研究成果及学术报告日益增多。尤其是与人们日益提高的生活质量戚戚相关的纳米氧化锌材料制备及应用。纳米氧化锌具有许多优良性能如压电性能、近紫外发射性、透明导电性、生物安全及适应性等,使其在非标柴油有害物质吸收、抑制食品污染菌、抗紫外线、压电材料、紫外光探测器、场效应管、表面声波、胎压、太阳能电池、气体传感器、生物传感器等领域有着广阔的发展前景而氧化锌复合材料的制备及研究也有着对人类生活不可估量的巨大作用。
溶胶。凝胶法的基本原理及应用
溶胶.凝胶法的基本原理及应用现状 溶胶.凝胶法(S01.Gel法,简称S.G法)就是以无机物或金属醇盐作前驱体,在液相将这些原料均匀混合,并进行水解、缩合化学反应,在溶液中形成稳定透明溶胶体系,溶胶经陈化,胶粒间缓慢聚合,形成三维空间网络结构的凝胶,凝胶网络间充满了失去流动性的溶剂,形成凝胶。凝胶经过干燥、烧结固化制备出分子乃至纳米亚结构的材料。溶胶.凝胶法就是将含高化学活性组分的化合物经过溶液、溶胶、凝胶而固化,再经热处理而成的氧化物或其它化合物固体的方法。近年来,溶胶-凝胶技术在玻璃、氧化物涂层和功能陶瓷粉料,尤其是传统方法难以制备的复合氧化物材料、高临界温度(P)氧化物超导材料的合成中均得到成功的应 1.基本原理 S01.Gel法的基本反应步骤如下: 1)溶剂化:金属阳离子M”吸引水分子形成溶 剂单元M(H20):+,为保持其配位数,具有强烈释放 H+的趋势。 2)水解反应:非电离式分子前驱物,如金属醇盐 M(OR)。与水反应。 3)缩聚反应:按其所脱去分子种类,可分为两类 a)失水缩聚 b)失醇缩聚 2.应用 由于溶胶.凝胶技术在控制产品的成分及均匀性方面具有独特的优越性,近年来已用该技术制成Li’ra02、“NbO,、PbTjO,、Pb(Zj孙)03和BaTjO,, 等各种电子陶瓷材料。特别是制备出形状各异的超导薄膜n0],高温超导纤维¨¨等。在光学方面该技术已被用于制备各种光学膜如高反射膜、减反射膜等和光导纤维、折射率梯度材料、有机染料掺杂型非线性光学材料等以及波导光栅、稀土发光材料等。在热学方面用该技术制备的SiO:一Ti0:玻璃非常均匀,热膨胀系数很小,化学稳定性也很好;已制成的InO,.SnO:(ITO)大面积透明导电薄膜具有很好 的热镜性能;制成的si02气凝胶具有超绝热性能等特点。 4研究展望 3.目前,对溶胶一凝胶法的研究主要集中在以下几 个方面: 1)在工艺方面值得进一步探索的问题:较长的制备周期;应力松弛,毛细管力的产生和消除,孔隙尺寸及其分布对凝胶干燥方法的影响;在凝胶干燥过程中加入化学添加剂的考察,非传统干燥方法探索;凝胶烧结理论与动力学以及对最佳工艺(干燥、烧结工艺)的探索。 2)和自蔓延法连用制备常规方法较难制备的新型纳米材料。例如 S01.GeI.EIsA(evaporati彻.induced se堆鹬sembly)制备一些具有纳米结构的功能性材料㈦。随着人们对溶胶.凝胶法的迸一步研究,溶胶.凝胶法一定能得到更为广泛的应用,在各个方面取得更大的进展。
溶胶-凝胶法制备纳米氧化锌
溶胶-凝胶法制备纳米氧化锌 摘要:纳米氧化锌是一种新型高功能精细无机材料,在光电器件、化工、医药等众多方面有着广泛的应用。本文结合国内有关溶胶-凝胶法制备纳米氧化锌方面的研究论文,设计了一种以醋酸锌为前驱物,草酸为络合剂,柠檬酸三铵为表面改性剂,无水乙醇、去离子水为溶剂,用溶胶--凝胶法制备纳米氧化锌的最优工艺过程,介绍、分析了溶胶--凝胶法制备纳米氧化锌的原理、工艺以及影响氧化锌粉体粒度、形貌及分散性的因素。 关键词:溶胶-凝胶法纳米氧化锌工艺影响因素 1 引言 氧化锌,俗称锌白,分子式为ZnO。纳米氧化锌为白色或微黄色晶体粉末,属六方晶系纤锌矿结构,晶格常数为a=3.24×10-10m,c=5.19×10-10m,为两性氧化物,密度为5.68g/cm3,熔点为1975℃,溶于酸和碱金属氢氧化物、氨水、碳酸铵和氧化铵溶液,难溶于水和乙醇,无味,无毒,无臭,在空气中易吸收二氧化碳和水。 纳米氧化锌是一种新型高功能精细无机粉料,其粒子尺寸在1~100nm之间。由于颗粒尺寸细微化,纳米氧化锌能产生其本体块状材料所不具有的表面效应、体积效应、量子尺寸效应和宏观量子隧道效应等,在磁、光、电、敏感等方面具有一些特殊性能。纳米氧化锌主要应用在橡胶、油漆、涂料、印染、玻璃、医药、化妆品和电子等工业,作为抗菌添加剂、防晒剂、光催化剂、气体传感器、图像记录材料、吸波材料、导电材料、压电材料、橡胶添加剂等[1]。 目前,纳米氧化锌的制备方法有很多,如沉淀法、微乳液法、溶胶- 凝胶法等,而溶胶--凝胶法因其制备均匀度高、纯度高及反应温度低、易于控制等优点,吸引了诸多的关注。 2 设计原理和反应原理 1.设计原理:溶胶--凝胶法制备纳米氧化锌。 溶胶--凝胶法是将金属有机或无机化合物经过溶液水解、溶胶、凝胶而固化,再经热处理而形成氧化物或其他化合物粉体的方法,其过程是:用液体化学试剂或溶胶为反应物,在液相中均匀混合并进行反应,生成稳定且无沉淀的溶胶体系。放置一定时间后转变为凝胶,经脱水处理,在溶胶或凝胶状态下成型为制品,再经过烧结固化制备出致密的氧化物材料[2,9]。溶胶--凝胶法制得的粉体粒度可
溶胶-凝胶法在制备纳米材料方面的应用
溶胶-凝胶法在制备纳米材料方面的应用 前言 纳米科技是一个跨学科的研究与开发领域,涉及纳米电子学、纳米材料学、纳米物理学、纳米化学、纳米生物学、纳米加工及表征等。纳米材料的合成与制备一直是纳米科学领域内 一个重要的研究课题,新材料制备工艺过程的研究与控制对纳米材料的微观结构和性能具有 重要的影响。最早是采用金属蒸发凝聚"原位冷压成型法制备纳米晶体,相继又发展了各种 物理、化学方法,如机械球磨法、非晶晶化法、水热法、溶胶-凝胶法等 溶胶-凝胶法是上个世纪6、70年代发展起来的一种制备无机材料的新工艺,近年来多 被用于制备纳米微粒和薄膜。溶胶-凝胶法具有反应条件温和通常不需要高温高压,对设备 技术要求不高,体系化学均匀性好,可以通过改变溶胶-凝胶过程的参数裁剪控制纳米材料 的显微结构等诸多优点。不仅可用于制备超微粉末和薄膜,而且成功应用于颗粒表面包覆, 成为目前合成无机纳米材料的主要技术,引起了材料科学技术界的广泛关注,是一个具有挑战性和应用前景非常广阔的领域。 1.溶胶-凝胶法的工艺原理: 溶胶凝胶法的工艺原理是:以液体化学试剂配制成金属无机盐或金属醇盐的前驱体,前驱体溶于溶剂中形成均匀的溶液(有时加入少量分散剂)加入适量的凝固剂使盐水解、 醇解或发生聚合反应生成均匀、稳定的溶胶体系,再经过长时间放置(陈化)或干燥处理使 溶质聚合凝胶化,再将凝胶干燥、焙烧去除有机成分、最后得到无机纳米材料。因此,也有 人把溶胶凝胶法归类为前驱化合物法。 根据原料的不同,溶胶凝胶法一般可分为两类,即无机盐溶胶凝胶法和金属醇盐水解法。(1)在无机盐溶胶凝胶法中,溶胶的制备是通过对无机盐沉淀过程的控制,使生成的颗粒 不团聚成大颗粒而生成沉淀,直接得到溶胶;或先将部分或全部组分用适当的沉淀剂沉淀出 来,经解凝,使原来团聚的沉淀颗粒分散成胶体颗粒溶胶的形成主要是通过无机盐的水解来 完成。反应式如下 (2)金属醇盐水解法通常是以金属有机醇盐为原料! 通过水解与缩聚反应而制得溶胶’首先将金属醇盐溶入有机溶剂! 加水则会发生如下反应: 式中M为金属R为有机基团,如烷基。经加热去除有机溶液得到金属氧化物材料。 2.溶胶-凝胶法的工艺过程: 溶胶凝胶法制备无机纳米材料过程主要包括5个步骤 (1)均相溶液的制备:溶胶凝胶法的第一步是制取包含醇盐和水均相溶液,以确保醇盐的 水解反应在分子级水平上进行。在此过程中,溶剂的选择和加入量是关键。 (2)溶胶的制备:在溶胶凝胶法中,最终产品的结构在溶胶形成过程中即已初步形成,后 续工艺均与溶胶的性质直接相关,因此溶胶制备的质量是十分重要的。有两种方法制备溶胶,一是先将部分或全部组分用适当沉淀剂先沉淀出来,经解凝,使原来团聚的沉淀颗粒分散成 原始颗粒。这种颗粒的大小一般在溶胶体系中胶核大小的范围内,因而可制得溶胶;另一种方法是由同样的盐溶液,通过对沉淀过程的严格控制,使首先形成的颗粒不致团聚为大颗粒 而沉淀,从而直接得到胶体溶液。 (3)凝胶化过程:缩聚反应形成的聚合物或粒子聚集体长大为小粒子簇,后者逐渐相互连 接成为一个横跨整体的三维粒子簇连续固体网络。在陈化过程中,胶体粒子聚集形成凝胶, 由于液相被包裹于固相骨架中,整个体系失去活动性,随着胶体粒子逐渐形成网络结构, 溶胶也从Newton体向Bingham体转变,并带有明显的触变性。在许多实际应用中,制品的成型就是在此期间完成的。
实验 溶胶凝胶法制备纳米二氧化钛实验
实验八溶胶-凝胶法制备纳米二氧化钛实验 一、实验目的 1、掌握溶胶-凝胶法制备纳米粒子的原理。 2、了解TiO 2 纳米粒子光催化机理。 二、实验原理 溶胶-凝胶法(Sol-Gel法)是指无机物或金属醇盐经过溶液、溶胶、凝胶而固化,再经热处理而成的氧化物或其它化合物固体的方法。 溶胶凝胶法制备TiO 2 纳米粒子是通过钛酸四丁酯的水解和缩聚反应来实现的,其分步水解方程式为: Ti(OR)n+H 2O Ti(OH)(OR) n-1 +ROH Ti(OH)(OR)n-1+H 2O Ti(OH) 2 (OR) n-2 +ROH …… 反应持续进行,直到生成Ti(OH)n. 缩聚反应: —Ti—OH+HO—Ti——Ti—O—Ti+H 2 O —Ti—OR+HO—Ti——Ti—O—Ti+ROH 最后获得氧化物的结构和形态依赖于水解与缩聚反应的相对反应程度,当金属-氧桥-聚合物达到一定宏观尺寸时,形成网状结构从而溶胶失去流动性,即凝胶形成。 三、原料及设备仪器 1、原料:钛酸正四丁脂(分析纯)、无水乙醇(分析纯)、冰醋酸(分析纯)、盐酸(分析纯)、蒸馏水 2、设备仪器:电磁搅拌器、恒温干燥箱、高温炉 四、实验步骤 以钛酸正丁酯[Ti(OC 4H 9 ) 4 ]为前驱物,无水乙醇(C 2 H 5 OH)为溶剂,冰醋酸(CH 3 COOH)为 螯合剂,从而控制钛酸正丁酯均匀水解,减小水解产物的团聚,得到颗粒细小且均匀的二氧化钛溶胶。 1、室温下量取10 mL钛酸丁酯,缓慢滴入到35 mL无水乙醇中,用磁力搅拌器强力搅拌10 min,混合均匀,形成黄色澄清溶液A。 2、将2 mL冰醋酸和10 mL蒸馏水加到另35 mL无水乙醇中,剧烈搅拌,得到溶液B,滴入2-3滴盐酸,调节pH值使pH=3。 3、室温水浴下,在剧烈搅拌下将溶液A缓慢滴入溶液B中。 4、滴加完毕后得浅黄色溶液,40℃水浴搅拌加热,约1 h后得到白色凝胶(倾斜烧瓶凝胶不流动)。 5、置于80 ℃下烘干,大约20 h,得黄色晶体,研磨,得到淡黄色粉末。 6、在 600 ℃下热处理2 h,得到二氧化钛(纯白色)粉体。 五、思考题 1、溶胶-凝胶法制备材料有哪些优点 2、纳米二氧化钛粉体有哪些用途 六、实验报告要求 实验报告按照学校统一模板书写,包括下列内容: 1、实验名称、目的和实验步骤。 2、解答思考题。
溶胶凝胶法制备氧化锌薄膜
一、所需试剂和实验仪器 试验中所需试剂(均为国药集团生产)及其作用: 二水合醋酸锌Zn(CH3COO)2?2H2O 金属前驱物 乙二醇甲醚CH3OCH2CH2OH 溶剂 无水乙醇CH3CH2OH 溶剂、清洗 异丙醇(CH3)2CHOH 溶剂 乙醇胺H2NCH2CH2OH 稳定剂 二乙醇胺HN(CH2CH2OH)2 稳定剂 九水合硝酸铝Al(NO3)3?9H2O 掺杂 丙酮CH3COCH3 清洗基片 浓盐酸HCL 清洗基片 去离子水H2O 清洗基片 制备薄膜的实验仪器设备 仪器型号用途 物理电子天平FA1104电子天平,测量前驱物及掺杂等物质 d=0.1mg,上海方瑞仪器 恒温磁力搅拌器78HW-1 型,金坛荣华仪器配制溶胶 台式匀胶机KW-4A 型台式匀胶机涂胶制备薄膜 中科院微电子研究所 电热恒温鼓风干燥箱DHG-9101.OSA 型预热处理薄膜 管式电阻炉SK-2-2-12 型预热和最终高温处理薄膜 上海实验电炉 测试仪器 X-射线衍射仪、高分辨透射电子显微镜、扫描电镜SEM、Hitachi-F4500荧光光谱仪等。 其它 石英硅片
二、实验步骤 (一)ZnO 前驱溶胶的制备 1、配制0.75 M 的溶胶: 用电子天平(精度为0.1mg)准确称取8.2621g 二水合醋酸锌放入大约30 mL 的乙醇溶剂中用具塞三角瓶盛放,用恒温磁力搅拌器搅拌并保持温度为70℃,10 分钟后加入4.60 mL的乙醇胺稳定剂,搅拌10 分钟后,待其冷却后在50 mL 容量瓶中用乙醇滴定,配制成0.75 M 的溶胶,最后在70℃的恒温磁力搅拌器上搅拌 1 小时后,形成均一透明的溶胶,至少静置48 小时后待用。在相同的条件下,分别用异丙醇(IPA),乙二醇甲醚(EGME)做为溶剂配制0.75 M 的ZnO 前驱物溶胶,至此我们配制了三种不同溶剂的ZnO 溶胶备用。 2、ZnO 薄膜的制备 我们选用石英片作为衬底。 (1)基片的清洗:采用石英片为基板,在涂膜前依次用浓盐酸、酒精、丙酮和丙酮酒精混合物以及去离子水在超声仪中清洗15 分钟,然后用于涂膜。 (2)薄膜的制备:我们在这次实验中采取四次涂膜。采用旋涂法进行涂膜,涂膜时先低转速600r/min,时间 6 s,然后高转速为3000 r/min,旋转时间为30s。涂膜结束后立即放入200o C 的烘箱中干燥10min,然后放入管式炉中进行500o C 热处理10 min。每一薄膜试样重复上述涂膜过程4 次。获得的ZnO 薄膜样品最后在600℃热处理 1 小时。 三、厚度、表面形貌及光学性质检测 1、X-射线衍射仪(X-Ray Diffraction) 从XRD 的结果可以确定晶体的物相、晶格常数和颗粒大小,还可根据峰的
氧化锌制备方法
将0.005 mol·L-1的NaOH乙醇溶液缓慢滴加到含有0.005 mol·L-1的Zn(NO3)2·6H2O乙醇溶液中. 将混合溶液转移至高压反应釜中, 在130℃下反应12 h, 将反应产物经二次去离子水、乙醇等洗涤后, 在130 摄氏度下干燥,即可获得纯ZnO纳米棒. 在 ZnCl2 溶液 (0.20 mol/L) 中加入一定量的 SDS, 搅拌下于 65 ℃将 Na2CO3 溶液滴加到该溶液中 (120 滴/min, n(Na 2CO 3 )/n(ZnCl2) = 2),恒温反应 0.5 h. 将反应液倒入聚四氟乙烯罐中, 在150~160 ℃进行水热反应 12 h, 自然冷却后离心分离, 用去离子水洗涤到无水Cl?离子, 再用无水乙醇洗涤 2~3 次, 50 ℃真空干燥 2 h, 300 ℃焙烧 3 h, 即制得 ZnO 纳米管. 将0. 1 L0. 1 mo l/ L二水合醋酸锌的乙醇溶液置于带冷凝管和干燥管的0. 5 L 圆底烧瓶中, 在80 ℃搅拌3 h, 不断收集冷凝物, 最后可获得0. 04 L 中间物和0. 06 L 冷凝物. 将中间物迅速用冷的绝对乙醇稀释至0. 1 L, 冷至室温, 得0. 1 mol/ L 中间产物. 氨水沉淀法制备纳米氧化锌 在水——乙醇介质中用氨水沉淀法制备出了纳米Zn(OH) 2 和ZnO材料,讨论了介质组成对沉淀产物ZnO微粒的粒径范围及形貌的影响,并研究出由Zn(OH)2分解为纳米ZnO的最佳干燥脱水条件为200℃、2h。表明本方法不需高温处理就可得到颗粒均匀且分布窄的ZnO纳米材料,粒径可达17~6nm。 一、试剂与仪器 主要原料为氯化锌、无水乙醇、氨水等,均为分析纯试剂。 仪器为微型滴定管、磁力搅拌器、恒温干燥烘箱。 二、试验方法 以水——乙醇为溶剂,其中醇的体积含量分别为0%(去离子水)、20%、60%、100%。将氯化锌、氨水配制成不同浓度的溶液(不同浓度是多少?)。取一定体积(一定体积是多少?)的氯化锌乙醇溶液于烧杯中,加以适当速度搅拌,不同浓度的氨水从微型滴管中缓慢滴入氯化锌乙醇溶液中,使之进行反应。控制氨水用量,调节pH值为7.0左右,确定滴定终点。反应得到的白色沉淀物,经抽滤洗涤后 自然风干即为Zn(OH) 2纳米粉,Zn(OH) 2 经干燥(200℃、2h)脱水后,为ZnO 纳米粉体。 三、不同乙醇浓度对ZnO粒径的影响 样品号 1 2 3 4 醇含量/%(体积分数 0 20 60 100 粒径范围/nm 286~46 100~31 38~14 17~6 这一结果表明,在此混合介质中,乙醇的存在对反应中生成的ZnO晶核的生长有明显的抑制作用,并且含量越高,这种抑制作用也越强。 四、氯化锌和氨水不同浓度下ZnO粒径大小 ZnCl2浓度/mol?L-1 粒径范围/nm 氨水浓度/%(体积分数)粒径范围/nm 0.5 32~12 10 32~14 1.0 25~15 15 25~15 2.0 34~10 25 16~7 氯化锌的浓度对ZnO的粒径影响不大,规律性不强;氨水的浓度对ZnO的粒径稍有影响,浓度增大,粒径是减小趋势,浓度为15%时,粒径为25~15nm,浓度为25%时,粒径为17~7nm。 五、该方法操作简单,条件温和,所用原材料成本低,过程易控制等,是制备
无机材料制备实验溶胶凝胶法合成莫来石
溶胶凝胶法合成莫来石(3Al 2O 3﹒2SiO 2)微粉 莫来石具有优异的高温强度、电绝缘性和化学稳定性,高的抗蠕变性和抗热震稳定性,低的热传导率和热膨胀系数及高温环境中优良的红外透过性等.莫来石陶瓷作为一种高温结构材料也受到越来越多的重视,此外,莫来石陶瓷在光学、电子等方面的应用,也引起人们的极大兴趣。莫来石有天然产物,但其含量和纯度均不能满足工业需要,为了获得高纯超细的莫来石原料,人们研究了一些特殊的合成工艺。如水解沉淀法,溶胶-凝胶法,成核生长法,喷雾热分解法,Al 2O 3和SiO 2超细粉末直接合成等. 溶胶凝胶法制备超细粉,是在液相中进行的,混合比较均匀,初始原料在液相中水解成水解产物的各种聚合体,各种聚合体进一步转化为凝胶.因凝胶比表面积很大,表面能高,与利用粉体之间固相反应的传统工艺相比,凝胶颗粒自身烧结温度低,其工艺上的优势对陶瓷粉体的工业生长具有重要的意义.粉料制备过程中无需机械混合,化学成分较均匀。由于转化温度低,可得超细粉末. 本实验采用溶胶-凝胶法合成莫来石微粉。 一、实验目的 1. 了解溶胶—凝胶法制备莫来石粉末的过程与原理; 2。 掌握溶液中铝含量的测定方法; 3。 掌握溶胶粘度的测定方法; 4. 学会用红外光谱初步测试无机粉末物相; 5. 掌握用激光粒度仪测试无机粉末粒度; 6. 掌握利用差热—热重联用仪研究样品在温度变化过程中所发生的物理化学变化。 二、实验原理 在正硅酸乙酯(TEOS )加入水,TEOS 开始水解反应,H + 取代了TEOS 中的烷基(—C 2H 5),随着水解的进行,发生聚合,小分子不断聚集成大分子,反应在宏观上就是粘度不断增大。由于溶胶中存在大量Al 3+ ,且Al 3+ 有一定夺氧能力,当溶胶聚合,逐渐形成三维网络时,大部分Al 3+ 进入Si —O 网络中,一部分Al 3+ 参与结构,形成复杂的—Si-O —Al —O 三维无轨网络。其水解缩聚机理如下: 2| | 33253 253 ||H O OR OR RO S i OC H Al NO RO S i OH C H OH Al NO OR OR ++ - +---++???→--+++
溶胶凝胶法
溶胶凝胶法 1 溶胶,凝胶法 溶胶,凝胶(Sol-Gel)技术是指金属有机或无机化合物经过溶胶,凝胶化和热处理形成氧化物或其他固体化合物的方法。其过程:用液体化学试剂(或粉状试剂溶于溶剂)或溶胶为原料,而不是用传统的粉状物为反应物,在液相中均匀混合并进行反应,生成稳定且无沉淀的溶胶体系,放置一定时间后转变为凝胶,经脱水处理,在溶胶或凝胶状态下成型为制品,再在略低于传统的温度下烧结。 2 溶胶凝胶法基本原理 溶胶,凝胶法的主要步骤为将酯类化合物或金属醇盐溶于有机溶剂中,形成均匀的溶液,然后加入其他组分,在一定温度下反应形成凝胶,最后经干燥处理制成产品。 2.1 水解反应 金属盐在水中的性质受金属离子半径,电负性,配位数等因素影响,如Si、Al 盐,它们溶解于纯水中常电离出Mn+,并溶剂化[3]。水解反应平衡关系随溶液的酸度,相应的电荷转移量等条件的不同而不同。有时电离析出的Mn+又可以形成氢氧桥键合。 水解反应是可逆反应,如果在反应时排除掉水和醇的共沸物,则可以阻止逆反应进行,如果溶剂的烷基不同于醇盐的烷剂,则会产生转移酯化反应,这些反应对合成多组分氧化物是非常重要的。 2.2 聚合反应 硅、磷、硼以及许多金属元素,如铝、钛、铁等的醇盐或无机盐在水解的同时均会发生聚合反应,如失水、失醇、缩聚、醇氧化、氧化、氢氧桥键合等都属于聚合反应,性质上都属于取代反应或加成反应。主要反应:,M,OH , HO,M, ? ,M,O,M,+H2O ;,M,OR + HO,M, ? ,M,O,M,+ROH 等。Okkerse等提出硅酸
在碱性条件聚合成六配位过渡态,Swain等则提出形成稳定的五配位的过渡态,由于硅酸盐的水解和聚合作用几乎同时进行,它的总反应过程动力学将决定于3个反应速率常数,使得在最临近的尺度范围内,中心Si原子可以有15种不同的化学环境,R.A.Assink等曾描述了这15种配位方式的关系。可见聚合后的状态是很复杂的[4-6]。 3 溶胶,凝胶法工艺过程 在Sol-Gel的全过程中,金属醇盐、溶剂、水及催化剂组成均相溶液,由水解缩聚而形成均相溶胶;进一步陈化成为湿凝胶;经过蒸发除去溶剂或蒸发分别得到气凝胶或干凝胶,后者经烧结得到致密的陶瓷体。同时,均相溶胶可以在不同衬底上涂膜,经过焙烧等热处理得到均匀致密的薄膜;也可以拉丝,得到玻璃纤维;以及均相溶胶经不同方式处理得到粉体[7]。 3.1 均相溶液的制备 这一步是制取包含醇盐和水的均相溶液,以确保醇盐的水解反应在分子级水平上进行。由于金属醇盐在水中的溶解度不大,一般用醇做溶剂,因为醇与醇盐溶液互溶,也跟水互溶,所以醇的加入量应适当,否则可能落入三元不混溶区。因为醇是醇盐水解产物,对水解反应有抑制作用,为保证起始溶液均匀性,对配置的混合液必须施以搅拌。为防止反应过程中易挥发组分散失,造成组成变化,一般需加回流冷凝装置。 3.2 溶胶的制备 一般将制备溶胶的方法分为聚合法和颗粒法。对醇盐来说,这两种方法的区别在于加水量的多少。在溶胶,凝胶法中,最终产品的结构在溶液中以初步形成,后续工艺与溶胶的性质直接相关,因此溶胶的质量是十分重要的。醇盐的水解和缩聚反应使均相溶液转变为溶胶,显然控制醇盐水解缩聚的条件是制备高质量溶胶的前
溶胶凝胶法制备纳米材料
利用溶胶凝胶法制备纳米材料的基本原理 学院:材料学院班号:1109102 学号:1110910209 姓名:袁皓 摘要:本文介绍了纳米材料的性能用途以及制备方法,主要是新兴的制备纳米材料低温工艺——溶胶凝胶法,在文中详细说明了溶胶凝胶法的类型和特征,重点描述了利用溶胶凝胶法制备纳米材料的类型,基本原理以及简略的操作流程。 关键词:纳米材料溶胶凝胶基本原理 一溶胶凝胶法的基本原理 溶胶凝胶(sol-gel)法是一种制备超细粉末的一种湿化学法,它是以液体的化学试剂配制成金属有机或无机化合物或者是金属醇盐前驱物,前驱物溶于溶剂中形成均匀的溶液,溶质与溶剂产生水解或是醇解反应,反应生成物在液相下均匀混合,均匀反应,生成稳定且无沉淀的溶胶体系,放置一段时间后或是干燥处理溶胶之后转变为凝胶,在凝胶中通常含有大量的液相物质,需要利用萃取或蒸发除去液体介质,并在远低于传统的烧结温度下热处理,最后形成相应物质化合物粉体,利用溶胶凝胶法还可以制备其他形态的材料包括单晶、纤维、图层、薄膜材料等。 表2-1 对于制备纳米材料的溶胶凝胶法类型和特征 1.1 溶剂化 能电离的前驱物-金属盐的金属阳离子M z+吸引水分子形成溶剂单元(M(H2O)n)z+(z 为M 离子的价数),为保持它的配位数而具有强烈的释放H+的趋势。 (M(H2O)n)z+==(M(H2O)n-1(OH))(z-1)++H+ 1.2 水解反应 非电离式分子前驱物,如金属醇盐M(OR)n(n 为金属M 的原子价,R 代表烷基),与水反应,反应可延续进行,直至生成M(OH)n。 M(OR)n+xH2O→M(OH)x(OR)n-x+xROH 1.3 缩聚反应 可分为失水缩聚:-M-OH+HO-M→M-O-M-+H2O 失醇缩聚:-M-OR+HO-M→-M-O-M+ROH
溶胶凝胶法
溶胶—凝胶法制备粉体 溶胶-凝胶法就是用含高化学活性组分的化合物作前驱体,在液相下将这些原料均匀混合,并进行水解、缩合化学反应,在溶液中形成稳定的透明胶溶体系,溶胶经陈化,胶粒间缓慢聚合,形成三维空间网络结构的凝胶,凝胶网络间充满了失去流动性的溶剂,形成凝胶。凝胶经过干燥、烧结固化制备出分子乃至纳米亚结构的材料。此方法的化学过程首先是将原料分散在溶剂中,然后经过水解反应生成活性单体,活性单体进行聚合,开始成为溶胶,进而生成具有一定空间结构的凝胶,经过干燥和热处理制备出纳米粒子和所需要材料。 一、基本原理 溶胶是指固体或胶体粒子均匀分散在溶液之中,固体粒子尺寸为1nm左右,含有103—109个原子,比表面积大。胶体粒子受到布朗运动的作用可以稳定持久地悬浮在液相之中,此外粒子的表面电荷引起的双电荷层使固体粒子更加均匀的分布在溶液之中。 凝胶是随着水分的蒸发,溶胶中固体粒子间聚合能量加强,逐渐失去流动而变成的半固态物质。分散在溶液中的固体粒子间吸引力与排斥力相当,使得凝胶中固态、液态都存在的高分散状态。 溶胶-凝胶法是以无机聚合反应为基础,以金属醇盐或无机金属盐作为前驱物,用水作为水解剂,有醇为溶剂来制备高分子化合物。在溶液中前驱物进行水解、缩合反应,形成凝胶。传统的溶胶-凝胶体系中,反应物通常是金属醇盐,通过醇盐缩水而得到溶胶。但由于稀土金属的醇盐易水解、成本高等问题,限制了溶胶—凝胶法在更多领域的应用。因此在很多领域中应用较多的是络合溶胶-凝胶法。该法在制备前驱液时添加强络合剂,通过可溶性络合物的形成减少前驱液中的自由离子,控制一系列实验条件,移去溶剂后得到凝胶,最后再通过分解的方法除去有机配体而得到粉体颗粒。 溶胶-凝胶过程具体包括以下两个反应过程: 1.水解反应是把阴离子取代成羟基,诱发综合反应,形成链状或网状交联的聚合物,金属盐类水解: ML + nH 2O → M(OH 2 )z+ n + L z- M(OH 2)z+ n → M(OH)(OH)(z-1)+ n-1 + H+ 2.缩聚反应是把OR或L和OH换去,转换成氧化态: M-OH + M-OH → M-O-M + H 2 O M-OH + M-OH → M-O-M + ROH 聚合程度决定于原颗粒的大小,而聚合速度取决于水解速率。如果水解反应速率大于缩聚反应速率,能够促进凝胶的形成。但在许多情况下,水解反应比缩聚反应快的太多,往往形成沉淀而无法形成稳定的均匀凝胶。要成功合成稳定的凝胶,关键在于降低络合物的水解速率,配制在pH值增大的条件下也足够稳定的前驱液。金属离子络合的目的是控制配位水分子在去离子反应中的水解速度,尽量减慢水解反应速度使缩聚反应完全。 二、影响因素
溶胶凝胶制备微晶玻璃
溶胶—凝胶技术制备微晶玻璃 摘要:玻璃的制备工艺多种多样,而用溶胶-凝胶法制备玻璃是近年来兴起的新工艺,本文简单介绍了利用溶胶-凝胶法制备微晶玻璃的状况。 关键词:溶胶凝胶;微晶玻璃;新型; 0 前言 玻璃是一种经过高温熔融得到的非晶态固体材料,具无规则结构的非晶态无机物,原子排列近似液体,近程有序,形状又象固体那样保持一定的形状。通常可按照生产工艺、成分和性能进行分类,具有各向同性、亚稳性、无固定熔点、可逆渐变性和连续性的特性。 玻璃的制备方法多种多样,根据不同的方法可分别从固态、气态、液态进行制备[1]。气态:气体辉光放电法、电解沉积法、溅射法、化学气相沉积法、物理气相沉积法;液态:急冷法(熔融冷却法);固态:粉末冶金法。这些方法都是较为传统的制备方法。随着制备技术的不断研究和发展,一些新的制备技术不断被应用于制备玻璃。如:辐照法、悬浮熔炼技术、溶胶-凝胶法、落管技术、粒子注入法、冲击波法、低熔点氧化物包裹法等。其中急冷法又可以细化出几种:喷枪法、锤砧法、离心法、压延法、单辊法、熔体沾出法和融滴法。 溶胶-凝胶合成法是在20世纪60年代中期作为制备玻璃、陶瓷材料的一种工艺发展起来的、在低温或温和条件下合成无机化合物和无机材料的重要方法。溶胶是指微粒尺寸介于1-100nm之间的固体质点分散于介质中所形成的多相体系;凝胶则是溶胶通过凝胶化作用(gelation)转变而成的、含有亚微米孔和聚合链的相互连接的坚实的网络,是一种无流动性的半刚性(semi-rigid)的固相体系。 1 特点 溶胶-凝胶法的优点:①通过溶液混合,易获得需要的均相多组分体系;②可大幅降低制备温度,在较温和的条件下合成出陶瓷、玻璃、纳米复合材料等功能材料;③可制备高纯或超纯物质,且可避免在高温下对反应容器的污染等问题;④溶胶或凝胶的流变性质有利于某种技术如喷射、旋涂、浸拉、浸渍等的实现。该制备方法存在的不足:①原料(金属醇盐)价格昂贵,醇的回收使技术和设备投资增加,且有机物危害健康,工业化生产有一定难度;②整个溶胶-凝胶过程通常需几天或几周的时间,时间较长;③凝胶中存在大量微孔,干燥过程中会逸出许多气体和有机物,干燥收缩大。 2微晶玻璃的制备 溶胶-凝胶法制备玻璃和制备薄膜、超细粉体的部分原理与技术相同或相似。即先由金属与醇类反应,醇氧化物分子中的有机基团与金属离子通过氧原子键合得到金属的醇氧化物[3]。醇氧化物一方面可溶于相似的醇溶剂中,另一方面当加入水时,醇氧化物与
溶胶凝胶法
溶胶-凝胶法 溶胶-凝胶法(Sol-Gel法,简称S-G法)就是以无机物或金属醇盐作前驱体,在液相将这些原料均匀混合,并进行水解、缩合化学反应,在溶液中形成稳定的透明溶胶体系,溶胶经陈化,胶粒间缓慢聚合,形成三维空间网络结构的凝胶,凝胶网络间充满了失去流动性的溶剂,形成凝胶。凝胶经过干燥、烧结固化制备出分子乃至纳米亚结构的材料。 溶胶-凝胶法由于其前驱物及其反映条件的不同可以分为以下几种制备方法。 l、金属醇盐水解法 该方法的基本过程是将醇盐溶于有机溶剂,然后在搅拌的同时缓慢加入蒸馏水的醇溶液,控制一定的pH值,经反应一定时间即可得到溶胶。溶胶的化学均匀程度一方面受到前驱液中各醇盐混合水平的影响,这与醇盐之间的化学反应情况密切相关;另一方面,每种醇盐对水的活性也有很大的差异。当金属醇盐之间不发生反应时,各种金属醇盐对水的活性起决定作用,反应活性的不同导致溶胶不均匀。添加有机络合剂是克服这些问题切实可行的办法,常用的络合剂有羧酸或β-二酮等添加剂。 2、强制水解法 该方法的基本过程是将将所要制备的金属氯化物加到氯化氢的水溶液中,将其加热到沸腾反应一段时间即得到对应的溶胶。这种方法在制备氧化物在氧化物阳极材料的制备中也得到了较为广泛的应用。 3.金属醇盐氨解法 4、原位聚合法及聚合螫合法 这种方法的作用机理是有机单体聚合形成不断生长的刚性有机聚合网络,包围稳定的金属螫合物,从而减弱各种不同离子的差异性,减少各金属在高温分解中的偏析 溶胶-凝胶法就是将含高化学活性组分的化合物经过溶液、溶胶、凝胶而固化,再经热处理而成的氧化物或其它化合物固体的方法。 ⑴Sol-Gel法的基本原理及特点 S01-Gel法的基本反应步骤如下: 1)溶剂化:金属阳离子M z+吸引水分子形成溶剂单元M(H2O)n x+,为保持其配位数,具有强烈释放H+的趋势。 M(H2O)n x+→M(H2O)n-1(OH)(x-1)+H+ 2)水解反应:非电离式分子前驱物,如金属醇盐M(OR)n与水反应。 M(0R)n+xH20=M(OH)x(OR)n-x,+xROH—M(OH)n 3)缩聚反应:按其所脱去分子种类,可分为两类 a)失水缩聚 —M—OH+HO—M—=—M—O—M—+H20 b)失醇缩聚 —M—0R+HO—M—=—M—O—M—+ROH
