13Copper Nanoparticle-Catalyzed Carbon-Carbon

DOI:10.1002/cssc.201100348
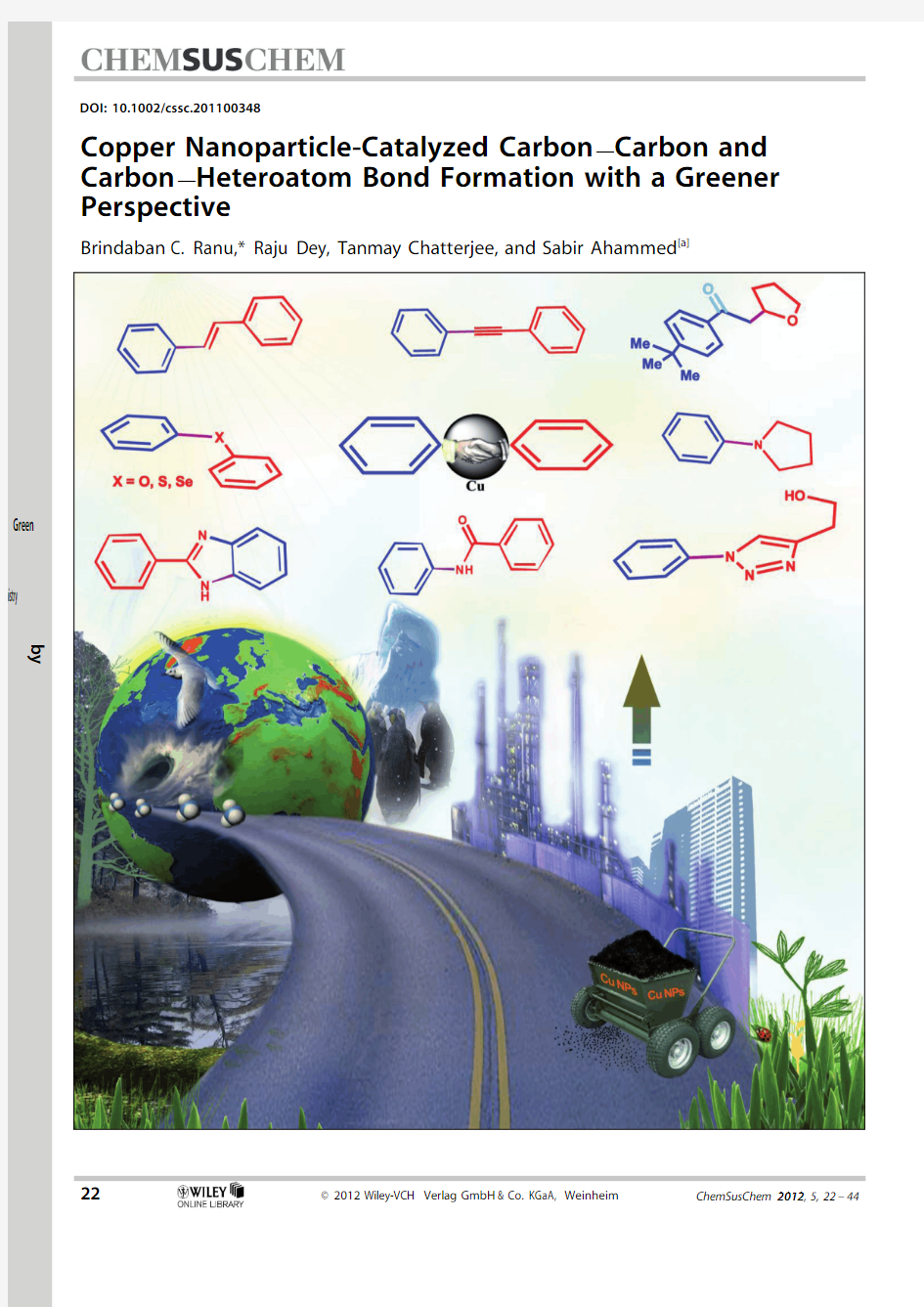
Copper Nanoparticle-Catalyzed CarbonàCarbon and CarbonàHeteroatom Bond Formation with a Greener Perspective
Brindaban C.Ranu,*Raju Dey,Tanmay Chatterjee,and Sabir Ahammed[a]
Green
Chemistry
by Nanocatalysis
1.Introduction
The division of larger particles into smaller ones with sizes be-tween1–100nm imparts unusual properties to these small species compared to those of bulk material.[1,2]These particles are called ultrafine particles[3]or nanoparticles(NPs),[4,5]where-as nanoclusters are defined as a material of at least one dimen-sion between1–10nm with a narrow size distribution.[6,7] Nanometer-sized single crystals are often referred to as nano-crystals.Nanoparticles,in general,are of great scientific interest as they effectively serve as a bridge between bulk materials and atomic or molecular structures.A bulk material should have constant physical properties regardless of its size;howev-er,at the nano-scale,size-dependent properties are often ob-served.The properties of materials change as their size ap-proaches the nano range,and the percentage of atoms at the surface of a nanomaterial becomes significant.For bulk materi-als larger than one micrometer(or micron),the percentage of atoms at the surface is insignificant with respect to the number of atoms in the bulk of the material.The interesting and sometimes unexpected properties of nanoparticles are, therefore,largely attributable to the large surface area of the material,which dominates the contributions made by the small bulk of the material.This characteristic change in the properties of nanoparticles compared to its counter bulk mate-rials may be attributed to the quantum size effect.With the decrease of particle size,the resultant surface-to-volume ratio increases considerably,which influences the surface-related properties of the materials.High surface area can provide better dispersion of the active sites and easy diffusion of the reactants and,hence,makes them suitable for catalytic activity. In general,the advantageous features of nanoparticles are: a)High surface-to-volume ratios of nanoparticles,which pro-vide a large number of active sites per unit area compared to their heterogeneous counterparts;[8]b)higher zeta potential for nanoparticles compared to bulk material,which prevent ag-gregation of nanoclusters;[9]c)low reduction potentials for nanoparticles,which facilitate oxidative addition in organome-tallic reactions;d)high reactivities of metal nanoparticles, which avoid the use of ligands;[10]and e)easy separation and recyclability of metal nanoparticles,which make them cost-ef-fective and minimize the chance of contamination of the cata-lyst with the product.[11]Thus,nanoparticles are endowed with several features of an efficient catalyst including green aspects useful for organic transformations.[12]
Although gold and silver received special attention mostly because of their ability to control economy,copper is also of considerable importance as an alternative to these metals. However,although copper occupies a position among transi-tion metals in the periodic table,metallic copper barely shows any catalytic activity like other members of this family.With the advance of nanotechnology and nanoscience,nanomateri-als received considerable interest because of their unique properties and applications in all branches of science.Similar to other nanomaterials,copper with nanometer-sized dimen-sions shows remarkable activity in applications from medicine to material to catalyst.
2.Copper Nanoparticles:Preparation and Characterization
Usually,two generic approaches are followed for the prepara-tion of nanoparticles:[13]top-down[14]and bottom-up[15] (Scheme1).The former is essentially the division of a massive solid into smaller portions.This approach involves the use of several methods,such as milling or attrition,chemical meth-ods,and volatilization of a solid followed by condensation of the volatilized components.In the bottom-up approach,the fabrication of nanoparticles is performed through condensa-tion of atoms or molecular entities in gas phase or in solution. This approach is gaining popularity in the preparation of nano-particles.
It is worth mentioning that dispersions of nanoparticles are intrinsically and thermodynamically metastable,[16]primarily be-cause of their high surface area.Uniform dispersion of the nanoparticles is dictated by the activation energy.When the activation energy is not sufficient to affect dispersion,an in-crease in nanoparticle size has been observed due to Oswald’s ripening.[17]Thus,highly dispersed nanoparticles are only kinet-ically stabilized[18]and cannot be prepared under
conditions [a]Prof.Dr.B.C.Ranu,R.Dey,T.Chatterjee,S.Ahammed
Department of Organic Chemistry
Indian Association for the Cultivation of Science
Jadavpur,Kolkata–700032(India)
Fax:(+91)33-2473-2805
E-mail:ocbcr@iacs.res.in G r e e n C h e m i s t r y b y N a n o c a t a l y s i s
that exceed some threshold.In addition,the use of surface-sta-bilizing agents has been used in synthesizing many nanomate-rials to hinder sintering,recrystallization,and aggregation.To prepare Cu,Cu2O,and CuO nanoparticles with well-defined morphology and chemical composition,both physical methods (chemical vapor deposition)and chemical methods(including sol–gel,hydrothermal,and electro-chemical deposition)have been followed.Some of them are discussed below.
2.1.Preparation of PEG-stabilized Cu Nanoparticles using microwave irradiation
Recently,Zhu et al.[19]reported the preparation of highly dis-persed copper nanoclusters through the reduction of a metal salt with suitable reducing agents(Scheme2)by using a solvo-thermal method.A basic aqueous solution of CuSO4was re-duced by N2H4·H2O in the presence of ethylene glycol as stabi-lizer,followed by microwave irradiation to give Cu nanoparti-cles.The energy-dispersive X-ray(EDX)spectrum(Figure1)and transmission electron microscopy image(TEM,Figure2)clearly indicate the formation of Cu nanoparticles with a size of4–6nm.These nanoparticles are highly reactive towards C(aryl)àS[20]and C(aryl)àSe[21]bond formation.
Henglein et al.observed that irradiation of a solution of copper perchlorate[Cu(ClO4)2]containing sodium formate led to the formation of colloidal copper[22]through reduction of Cu2+by both,solvated electrons and CO2generated during radiolysis.
2.2.Preparation of polyvinylpyrrolidone-coated Cu2O nano-particles
Chen and Kong et al.[23]developed a simple method for the synthesis of polyvinylpyrrolidone-coated Cu2O nanoparticles (Scheme3).The nanoparticles were capped inside the polymer matrix to restrict agglomerization.In a typical experimental procedure,an aqueous solution of copper acetate(Cu(OAc)2) was added to an aqueous mixture of sodium borohydride con-taining a polyvinyl polymer(40kDa)while stirring vigorously at room temperature.After2–3h,the solution turned dark brown.Then,the solution was centrifuged at10000rpm for 10min,and the precipitate was dried under vacuum.
The particle size distribution of PVP-coated Cu2O nanoparti-cles is20?10nm,and the catalyst is highly active towards azide–alkyne click reactions in water.
Academy of Sciences and
recipient of the J.C.Bose
N.S.Narasimhan Award
(2009)medal of the Chemical Presently,he is pursuing his Benign Solvents,Reagents,
for the Cultivation of Sci-
supervision of Prof. B.C.
Green
Chemistry
by
Nanocatalysis
B.C.Ranu et al.
2.3.Solvothermal preparation of CuI nanoparticles
Xu and Feng et al.[24]reported a solvothermal method for the preparation of CuI nanoparticles by the reaction of [Cu(dmg)2](DMG =dimethylglyoxime)and KI in an autoclave (Scheme 4)
with ethanol as solvent.dmgH and Cu(OAc)2·H 2O were added into absolute ethanol in sequence,which was stirred at 08C for 30min to obtain a brown precipitate of Cu(dmg)2.The collect-ed precipitate was dispersed in absolute ethanol followed by addition of KI,and the mixture was stirred vigorously for 2h.Then,the mixture was transferred into a teflon-lined stainless steel autoclave.
The autoclave was sealed and heated at 1808C for 6h.The black precipitate thus obtained was centrifuged and washed with ethanol and deionized water,which was followed by vacuum drying for 12h at room temperature to give CuI nano-particles (30–80nm)of high purity.This catalyst is very efficient and selective for the synthesis of phenols,anilines,and thio-phenols from aryl halides in aqueous
solution.
Scheme 1.Conventional routes for the preparation of
nanoparticles.
Scheme 2.Preparation of PEG-stabilized Cu 0nanoparticles using microwave
irradiation.
Figure 1.EDX spectra of copper nanoparticles (adopted from Ref.
[20]).
Figure 2.TEM image of the Cu nanoparticles showing spherical particles with diameters in the range 4–6nm (adopted from Ref.
[20]).
Scheme 3.Preparation of PVP-coated Cu 2O nanoparticles by using a strong reducing agent at room
temperature.
Scheme 4.Preparation of CuI nanoparticles at very high temperatures in an autoclave.
G r e e n C h e m i s t r y b y N a n o c a t a l y s i s
Copper-Catalyzed Bond Formation with a Greener Perspective
2.4.Core–shell nanoparticles consisting of Cu/Cu 2O
Hyeon and coworkers [25]demonstrated a straightforward and efficient method for the preparation of core–shell nanoparti-cles consisting of Cu/Cu 2O (Scheme 5)and the subsequent use of the nanocatalyst in the Ullmann-type amination of aryl
chlorides.In an experimental procedure,Cu(acac)2dissolved in oleyl amine was slowly heated to 2308C and held at this tem-perature for 6h,which produced a red colloidal solution.A TEM image showed that uniform 15nm-sized Cu nanoparti-cles were formed.When these Cu nanoparticles were exposed to air,the color of the nanoparticle solution turned blue.
The TEM image showed that the size and shape of the nano-particles remained practically unchanged after oxidation in air.The XRD pattern (Figure 3)suggested the formation of a Cu 2O shell from the copper core.The TEM and high resolution TEM
(HRTEM)image of an oxidized sample indicated the formation of polycrystalline Cu 2O on the surface.2.5.Supported nanoparticles
Because of the small size of metal nanoclusters and their solu-bility in reaction media,it is not always easy to separate them from the solution.Thus,nanoparticles are anchored onto a solid surface to achieve an easy separation,and a few proce-dures are described below.
2.5.1.Silica-supported Cu nanoparticles
Highly dispersed Cu nanoparticles supported on silica were prepared by using the straightforward and convenient precipi-tation–gelation technique (Scheme 6)reported by Chen and Xia et al.[26]An aqueous NaOH solution was added dropwise to a solution of Cu(NO 3)2at a constant rate while stirring vigo-rously to form a precipitate.Next,a calculated amount of col-loidal aqueous silica solution was added to the solution of the precipitate to form a gel,and the gel was allowed to age at 363–373K for 4h.Finally,the slurry of the gel was filtered,thoroughly washed with hot distilled water,dried at 1308C overnight,and calcined at 4008C under air for 3h.
To produce silica-supported Cu nanoparticles,the immobi-lized CuO/SiO 2was autoclaved in a stainless steel bomb at 3008C for 3h in an H 2stream.
X-ray photo electron spectra (XPS,Figure 4)clearly indicate the presence of Cu 2+in the catalyst after calcination,and it was completely converted to Cu 0after reduction.Cu/SiO 2cata-lysts prepared by using this method showed high selectivity (>98%)towards glycerol hydrogenolysis.
2.5.2.Mesoporous silica-supported Cu nanoparticles
Copper(II)sulfate was added to the basic aqueous solution of b -cyclodextrin.To this clear blue solution,b -methyl cyclodex-trin was added while stirring.After filtration,the filtrate was poured into a vigorously stirred prehydrolyzed silica gel (tetra-methyl orthosilicate)in an acidic medium.This mixture was kept in an open flask at room temperature for approximately one week.The resulting silica hybrid composites were subject-ed to calcination at 5008C to provide mesoporous silica con-taining Cu nanoparticles with an average pore size of 6–8nm.[27]
2.5.
3.Deposition of Cu on activated carbon
A solution of CuI in absolute ethanol (30mL)was refluxed for 4h in the presence of activated carbon under an inert atmo-sphere to produce Cu nanoparticles deposited on carbon.[28]The resulting materials were washed with ethanol and dried under vacuum.Inductively coupled plasma analysis
revealed
Scheme 5.Preparation of Cu 2O-coated Cu
nanoparticles.
Figure 3.XRD pattern of Cu 2O coated Cu
nanoparticles.
Scheme 6.Preparation of silica-supported Cu
nanoparticles.
Figure 4.XPS spectra (Cu 2P)of a)calcined sample and b)calcined sample after reduction.
Green Chemistry by Nanocatalysis
B.C.Ranu et al.
that the copper content of the catalyst was approximately 9.97%.
However,the CuI crystals tend to deposit on the activated carbon because of the low solubility of CuI in ethanol.The SEM image (Figure 5)showed the morphology of CuI crystals.
The poor solubility of CuI under the reaction conditions re-sults in a low concentration of Cu +in the solution.Thus,Cu +produces Cu 0nanoparticles (Scheme 7)and Cu 2+species through a disproportionation reaction,and the resultant Cu 2+is reduced by the iodide present in the reaction mixture to re-produce CuI.The above sequences of reactions are repeated continuously during the process to generate Cu/CuI,which plays an active role in the catalysis of organic reactions.
2.5.4.Magnetically separable Cu catalysts
Chi and Hur et al.developed a method for the preparation of Cu 3N nanoparticles supported on mesoporous superparamag-netic silica microspheres (Scheme 8).[29]In an experimental pro-cedure,Fe(NO 3)3·9H 2O,dioctyl ether,and a nonionic surfactant (Igepal CO-520)in ethanol were added to mesoporous silica,followed by stirring and sonication for 10min.The reaction
mixture was heated to 908C and centrifuged.The solid mass was dried in an oven at 808C for 24h.This was further an-nealed at 7008C in an NH 3atmosphere for 6h to give the Fe 3N-SiO 2matrix.This Fe 3N-SiO 2matrix was sonicated with Cu(OAc)2·H 2O,cetyl trimethylammonium bromide (CTAB),and octane in methanol for 10min followed by addition of 1-buta-nol to the solution.The reaction mixture was heated to 808C,and the resultant colloidal microspheres were separated by performing centrifugation and dried at 808C for 24h.This was further annealed at 2508C in an NH 3atmosphere for 6h to produce the Cu 3N-Fe 3N-SiO 2catalyst.
2.6.Characterization of nanoparticles
Several techniques are used for the characterization of nano-materials.Among them,TEM analysis is used to determine shape and size (diameter)of the nanoparticles (Figure 2).EDX of the selected area of nanoparticles gives an indication of the composition (Figure 1).The oxidation state of the metal pres-ent in the material can be easily determined by using XPS (Figure 4).The phase purity of the bulk material of the nano-particles can be determined by using XRD (Figure 3)with com-parison of those reported earlier.For nanomaterials with a comparatively large size,scanning electron microscopy images (SEM,Figure 5)gives information regarding its morphology.UV spectroscopy also gives useful information of the nature of nanoparticles.
3.Copper Nanoparticles as Catalysts
Because of growing environmental concerns,copper has re-ceived tremendous attention in organic synthesis owing to its environmentally benign character,easy availability,and low https://www.360docs.net/doc/1011790454.html,e of copper in catalysis has dramatically changed the scenario in organic transformations.A number of outstanding catalytic systems have been developed in recent years,which provide excellent stereo-,regio-,chemo-,and enantioselectivity in many organic reactions,such as formation of C(aryl)àC(aryl),C(aryl)àN,C(aryl)àO,C(aryl)àS,and C(aryl)àSe bonds as well as many other useful transformations.A brief account of all these reactions will be presented.
3.1.Carbon àcarbon bond formation 3.1.1.Ullmann coupling reaction
Ullmann coupling,named after Fritz Ullmann,between two aryl halides catalyzed by stoichiometric amounts of copper or copper bronze alloy producing biaryls is well-known in organic synthesis.[30]The traditional Ullmann reaction involves harsh re-action conditions and elevated reaction temperatures.More-over,lower yields of products observed with substituted aryl halides are another limitation for this reaction.Since its discov-ery a century ago,[31]many improvements and alternative pro-cedures have been introduced.Recently,Maitra et al.[32]report-ed a Cu nanoparticle-catalyzed Ullmann coupling (Scheme
9).
Figure 5.SEM image of Cu and CuI particles on activated
carbon.
Scheme 7.Formation of Cu nanoparticles on activated
carbon.
Scheme 8.Cu 3N nanoparticles supported on a mesoporous superparamag-netic silica.
G r e e n C h e m i s t r y b y N a n o c a t a l y s i s
Copper-Catalyzed Bond Formation with a Greener Perspective
Uncapped and undefined macrosized copper powder showed only 43%conversion of iodobenzene to biphenyl in
5h under the experimental conditions.However,use of Cu nanoparticles (66nm)prepared by applying citrate capping showed 88%conversion,and it was further increased to 95%when finer Cu nanoparticles (8nm)were used.Further division of Cu nanoparticles did not further improve the performance.The reported reaction involves the formation of an aryl–copper intermediate (A)in the first step (Scheme 10),which then
reacts with another aryl halide molecule through oxidative ad-dition to form intermediate (B),which provides the biaryl on reductive elimination.
This procedure of Ullmann coupling using environmentally benign Cu nanoparticles is an attractive alternative to the ex-isting procedures.
3.1.2.Suzuki reaction
Cross-coupling of aryl halides with aryl boronic acids leading to biaryls was discovered by Suzuki and coworkers [33]and is one of the most powerful synthetic tools in organic synthesis.Because of its ease of operation and general applicability,Suzuki coupling has become the method of choice for labora-tory and industrial applications.Palladium-,nickel-,and iron-based catalysts are usually used for this reaction.Rothenberg et al.[34]reported a Cu nanoparticle-catalyzed Suzuki cross-cou-pling reaction (Scheme 11).Colloidal Cu nanoparticles showed significant activity to afford quantitative yields after 8h at 1108C.
However,this procedure is limited to aryl iodides only,and other halides are not reactive.
3.1.3.Stille reaction
Cross coupling between aryl halides and organotin derivatives is known as Stille coupling.[35]Li and Zhang et al.developed a versatile reaction (Table 1)catalyzed by Cu 2O nanoparticles using an ionic liquid,tetrabutylammonium bromide (TBAB),as reaction medium and KF as a promoter in the presence of P(o -tol)3as a ligand.[36]
The reaction is compatible with several substituents present on the aromatic rings.The reaction of other aryl halides includ-ing less reactive aryl chlorides proceeds well with the Cu 2O NPs/P(o -tol)3/TBAB reagent system.Moreover,the use of ionic liquid as solvent and inexpensive and readily available Cu 2O as reusable catalyst make this protocol an attractive green alter-native to the existing procedures for this reaction.However,the catalytic activity of the reusable system was reduced in the second run of the coupling of the deactivated aryl bromide.3.1.4.Heck reaction
The use of metal alloy as catalyst is well-known in organic syn-thesis.[37]However,the active catalytic species in the reaction were not precisely determined.Recently,Calo and
coworkers
Scheme 9.Ullmann coupling reactions of aryl iodide.Reagents:Iodoben-zene (0.5mmol),Cu NPs (1.6equiv.),DMSO (1
mL).
Scheme 10.Probable reaction mechanism for Ullmann coupling
reactions.
Scheme 11.Suzuki coupling reaction between aryl iodide and phenyl boronic acid.Reagents:Iodobenzene (0.50mmol),phenyl boronic acid (0.75mmol),K 2CO 3(1.5mmol),catalyst (2mol %),DMF (12.5mL).
Halide
Time [h]I 12Br
24Cl 60[a]Reagents and reaction (0.4mmol),Cu 2O NPs TBAB (1.5g);125-130Green Chemistry by Nanocatalysis
B.C.Ranu et al.
observed that when copper bronze alloy was treated with iodobenzene,Cu nanocolloids (%4nm)were formed,which played the active role in catalysis.[38]An efficient Heck reaction between activated alkenes and aryl iodides/bromides catalyzed by these colloidal NPs (Table 2)in TBAB was reported.[39]
After each cycle,the efficiency of the catalyst increased gradually and was maximum for the sixth cycle,as shown in the product conversion chart (Figure 6).Therefore,it was sug-gested that the tin metal present in the bronze was gradually consumed by aryl halide and small particles of metallic copper were generated.Agglomerization of these small particles ulti-mately led to the formation of Cu nanoparticles stabilized by TBAB.In addition,halide ions play a crucial role in the forma-tion of nanoparticles.[40]
3.1.5.Sonogashira cross-coupling
The coupling between aryl halides and terminal alkynes known as Sonogashira coupling [41]is usually performed by using a va-riety of palladium or palladium–copper mixed-metal catalysts.Rothenberg et al.[42]showed that Cu nanoparticles efficiently catalyze the reaction between phenyl acetylene and a variety of iodo-and bromo-arenes (Table 3).Significantly,these Cu
clusters can be recycled up to three cycles without any loss of activity,giving a final turnover number of 73.Surprisingly,the state of the catalyst after the third cycle and any data of leach-ing of the catalyst were not available.
A mechanism was proposed based on oxidative addition and reductive elimination.Phenylacetylene reacts with a Cu cluster,forming an [alkenyl–Cu]cluster (species 1),which reacts with the aryl halide to form 2,and this intermediate fi-nally leads to the product by reductive elimination as depicted in Scheme 12.
Significantly,the positive charge created during oxidative addition is shared among the copper atoms in the cluster,
Yield [%]Product
8190
[a]Reagents and reaction conditions:Aryl halide (1mmol),butyl (1.2mmol),TBAA (1.5mmol),TBAB (3g),copper bronze (3mol %);16h.[b]The corresponding aryl bromide was
used.
Figure 6.Recyclability chart of the coupling reaction of p -iodoanisole and butyl acrylate catalyzed by TBAB/Cu nanoparticles (adopted from ref.[38]).
Sonogashira reaction of terminal alkynes with aryl halides.Yield [%]Product
849999[b]
[a]Reagents and reaction conditions:Aryl halide (0.25mmol),acetylene (0.38mmol),TBAA (0.40mmol),Cu nanoclusters (1.2DMF (2.5mL);1108C,
24h.corresponding aryl bromide was Scheme 12.Probable mechanistic path for the Sonogashira reaction.
G r e e n C h e m i s t r y b y N a n o c a t a l y s i s
Copper-Catalyzed Bond Formation with a Greener Perspective
which is contrast to monoatomic complexes.This facilitates product formation and reductive elimination steps.
3.1.6.Difunctionalization reaction
Park et al.[43]reported a PVP-stabilized Cu2O nanocubes cata-lyzed difunctionalization of vinyl arenes with cyclic ethers through CàH activation(Table4).
The catalytic oxyalkylation of various substituted styrenes was performed by refluxing a mixture of styrene,catalyst,and THF.The process is simple,and a wide range of oxyalkylated vinyl arenes are obtained in excellent yields with high regio-selectivity.The high chemical reactivity of the nanoparticles plays an important role in the improvement of the yield of the reaction.
3.1.7.Arylation of active methylene compounds
Kidwai et al.reported a CuO nanoparticle-catalyzed arylation of an active methylene compound(Table5).[44]C-Arylation of ace-tylacetone and dimethyl malonate with a variety of substituted iodobenzenes provided the corresponding products in high yields.
The CuO nanoparticles show superior reactivity over Cu(OAc)2,parent CuO,and even Cu nanoparticles with respect to the reaction yield and reaction time.After the completion of the reaction,CuO nanoparticles were recovered by centrifu-gation and reused four times.However,no data regarding leaching of the Cu catalyst was provided.3.1.8.Three-component condensation
Cu nanoparticles catalyzed the three component coupling re-action of aromatic aldehydes,amines,and alkynes through CàH activation to form propargyl amines(Table6),[45]which are
Difunctionalization reaction between alkene,air,and ether.
Yield
[%]
Product
98
100
95
[a]Reagents and reaction conditions:Styrene(13.9mmol), (1mol%),THF(10mL);0.3MPa,air.
Yield
[%]
Product
80
81
[a]Reagents Active methylene (3mmol),aryl(10mol%),Cs DMSO(2mL);
Table6.Coupling aldehyde,amine,and CuO nanoparticles.
Product Yield
[%]
Product
87
89
[a]Reagents and reaction conditions:Aldehyde(1.0mmol), amine(1mmol),phenyl(1.5mmol),Cu NPs(15mol (5mL);100–1108C,3.5–7
Green Chemistry by Nanocatalysis
B.C.Ranu et al.
very useful precursors to many biologically active compounds and natural products.The catalyst was recycled up to seven times.
Copper was the best among other metal nanoparticles,in-cluding nickel,silver,and gold.Nevertheless,copper is less ex-pensive and environmentally benign.
3.2.Carbonànitrogen bond formation
CàN bond formation is a very useful process,as it provides a powerful tool for the synthesis of numerous compounds with biological,material,and therapeutic importance.
3.2.1.Anilines from aryl halides in aqueous solutions Aromatic amines are of considerable importance,as they are used as building blocks for many structurally complex and bio-logically active molecules.The amines are also useful for the polymer,dye,and pharmaceutical industry.Recently,Xu and Feng[24]reported a CuI nanoparticle-catalyzed selective amina-tion(Table7)of aryl halides in water under mild conditions. The reactions proceeded in the presence of both,electron withdrawing(EWG)and electron donating(EDG)substituents present on the aromatic ring,providing high yields of products.
3.2.2.N-Arylation reaction
N-Arylation of amines through CàN cross-coupling reaction with aryl halides is a useful process for preparing aryl amines.[46]In conventional procedures,CàN bond forming re-actions usually require stoichiometric amounts of copper re-agent,[47]which makes waste disposal a serious issue for large scale reactions.To overcome this problem,methods utilizing catalytic amounts of metal salts have been developed.[48] Several ligands have been used in palladium and copper com-plexes for the amination of aryl halides.[49]However,Punniya-murthy et al.[50]have developed a CuO nanoparticle-catalyzed ligand-free CàN cross-coupling reaction of amines with iodo-benzene(Table8)in excellent yield.This catalytic system is also effective for the reactions of bromobenzenes and chloroben-zenes with substituted anilines.
A variety of alkyl amines,such as benzyl amine,furfuryl amine,n-butyl amine,and cyclohexyl amine,and N-heterocy-clic compounds,such as pyrrolidine,piperidine,and morpho-line,gave85–93%yields.A variety of substituted iodoben-zenes also underwent high yielding reaction with aniline.After completion of the reaction,the CuO nanoparticles were sepa-rated by performing centrifugation and reused for three suc-cessive runs without significant loss of activity.
The reaction was proposed to proceed through a catalytic cycle involving formation of CuàN bonds through insertion fol-lowed by oxidative addition of the aryl halide and reductive elimination as depicted in Scheme 13.
Amination of aryl halides.
Halide Yield
[%]
Product Halide
I97
Br87
Br91[b]
I89
[a]Reagents and reaction conditions:Aryl bromide(1.0mmol),
(3.0mol%,X=Br;1.5mol%,X=I),n Bu4NOH(3mmol),28%aqueous
(5equiv.,X=I;10equiv.,X=Br);(RT,X=I;808C,X=Br),N2atmosphere, 24–48h for X=I and Br,10equiv.NH
CuO NP-catalyzed N-arylation reactions.
Yield
[%]
Product
92
98
70
[a]Reagents and reaction conditions:Aniline(2.5mmol),iodobenzene
(2mmol),CuO NPs(1.2mol%),KOH(2mmol),
DMSO(4mL);
Scheme13.Proposed mechanism for N-arylation reactions.
G
r
e
e
n
C
h
e
m
i
s
t
r
y
b
y
N
a
n
o
c
a
t
a
l
y
s
i
s
Copper-Catalyzed Bond Formation with a Greener Perspective
3.2.3.Arylation of aromatic heterocycles
Li et al.[51]introduced a Cu 2O nanoparticle-catalyzed solvent-free procedure for the N -arylation of nitrogen-containing het-erocycles (Table 9),such as imidazole,triazole,and indoles,by
using aryl and heteroaryl halides.The cubic Cu 2O nanoparticle works best for the N -arylation reaction compared to other structures,such as bulky,octahedral,and spherical nanoparti-cles.Cu 2O nanoparticles show high efficiency for the N -aryla-tion of aryl halide and heteroaryl halide in neat conditions using tetra-n -butylammonium fluoride (TBAF)as a base at 1408C.
3.2.
4.Arylation of amides
The N -arylation of amides is of considerable significance,as the products are core units of many natural products and bio-logically active molecules (Figure 7).The Goldberg reaction [52]
is frequently used for preparing N -arylamides.However,these reactions are performed at elevated temperatures (>1508C).Several palladium-and copper-based catalysts with various types of ligands were also used for the reaction.[53]Cu 2O nano-particles in poleyethylene glycol (PEG)are efficient and recycla-ble catalysts for the amidation of aryl iodides (Table 10).[54]No ligand,additive,or cocatalyst is required for this reaction.
A disadvantage of this methodology is the use of expensive aryl iodides,which limits its use in industrial processes.To overcome this problem,Xianwen and coworkers [55]introduced a Cu 2O nanoparticle catalyst in combination with 10mol %di-methylethylenediamine (DMEDA),which facilitates amidation with less reactive aryl bromides and chlorides (Table 11)in high yields.
The steric effect was determined to be more significant than the electronic effect.The reaction is highly chemoselective,as benzamide is selectively arylated in the presence of free aro-matic amines.Secondary aliphatic amides afforded the desired products in high yields.The reaction of less reactive aryl chlor-ides was successfully performed in this catalytic system.The catalyst was reused in the next reaction without any loss of activity.
3.2.5.Intramolecular carbon ànitrogen bond formation Benzimidazole moieties play an important role in the recogni-tion of biological and therapeutic activities.Development of general methods for the synthesis of these compounds is highly appreciated in drug discovery and medicinal chemistry.Punniyamurthy et al.utilized an intramolecular C àN bond for-mation using CuO nanoparticles as the catalyst for the synthe-sis of substituted benzimidazoles (Table 12).[56]
A variety of functional groups are compatible with this pro-cedure.High yields of products are achieved irrespective of number and nature of R,R 1,or R https://www.360docs.net/doc/1011790454.html,e of
recyclable
Figure 7.Examples of some biologically active compound containing C àN bonds.
-arylation of nitrogen-containing aromatic heterocycle.Yield [%]
Product
77
63
[a]Reagents and conditions:Heterocyclic (0.5mmol),aryl halide Cu 2O NPs (10mol nanthroline (20mol 140–1458C,24–48h.
Arylation of amides using aryliodide.Yield [%]
Product
80
70
75
[a]Reagents and reaction Amide (1.2mmol),(1mmol),Cu 2O NPs PEG-4000(1g);1208C,3–16h.
Green Chemistry by Nanocatalysis
B.C.Ranu et al.
copper catalyst significantly reduces the E -factor (the ratio of the amount of waste to the amount of product),which makes this a greener procedure in comparison to other available methods.[57]
3.2.6.Aziridination reaction
In 1967,Kwart and Kahn described a copper–bronze alloy-cata-lyzed aziridination of cyclohexene (Scheme 14)with benzene sulfonyl azide.[58]Although the methodology has serious limita-tions with regard to yields of the products (15%),this concept led to the future development of efficient aziridine synthesis.
Recently,Kantam et al.reported an aziridination reaction (Table 13)catalyzed by alumina-supported Cu nanoparticles in moderate to high yields.[59]
Use of environmentally friendly and recyclable heterogene-ous copper nanocatalysts at room temperature is a significant advantage of this procedure.
3.2.7.Click reaction
Click chemistry is a newer approach to the synthesis of drug-like molecules that can [60]accelerate the drug discovery pro-cess by utilizing practical and reliable reactions.[60]Sharpless and coworkers [61]defined a click reaction as one that is wide in scope and easy to perform,uses readily available reagents,and is insensitive to oxygen and water.[62]In several cases,water is the ideal reaction solvent,providing the best yields and high-est rates.
11.Arylation of amide using aryl chloride,bromide and iodide.Halide Yield [%]Product
Halide Cl [b]82Br I 95I Br Cl [b]815267I Cl [b]
8078
Br [a]Reagents and reaction conditions:Amide (0.6mmol),aryl mide (0.5mmol),Cu mol %),DMEDA (10mol %),(0.75mmol.),toluene C,10h.[b]Neat conditions for 25h.
Table 12.CuO nanoparticle-catalyzed synthesis of substituted zoles.[a]
Product
Time [h]Yield [%]4
94
492
1888
Reagents and reaction conditions:Reactant (1mmol),
KOH (1.5mmol),DMSO (1mL);1108C,4–18h,air.
Scheme 14.Synthesis of aziridine through reaction of benzene sulfonyl azide with cyclohexene.
nanoparticle-catalyzed azidirination reactions.Product
Time [h]Yield [%]3
953
74
3
754
75
and reaction catalyst (4.5mol G r e e n C h e m i s t r y b y N a n o c a t a l y s i s
Copper-Catalyzed Bond Formation with a Greener Perspective
Sharghi et al.[28]developed a one-pot procedure for the syn-thesis of1,2,3-triazole derivatives through a three-component coupling reaction of terminal alkynes,benzyl or alkyl halides, and sodium azide in the presence of1mol%copper/carbon (Cu/C)nanoparticles.[63]Cu nanoparticles supported on activat-ed charcoal offers superior catalytic activity and1,4-regioselec-tivity towards the[3+2]Huisgen cycloaddition in water.[64]
The solvent plays a significant role in this reaction.The reac-tion of benzyl bromide,phenylacetylene,and sodium azide in the presence of1mol%of Cu/C in water provided the1,4-di-substituted triazole product in91%yield by stirring for45min at1008C in an open system(Table14).
Benzyl halides with an electron-donating group on the phenyl ring underwent facile reactions with high yields,where-as substitution of the electron-withdrawing group led to lower yields of the products.The reaction of an aliphatic acetylene, such as1-hexyne,requires longer reaction times.Internal al-kynes remained inert in this reaction.The reaction offers a good scope for the synthesis of new azacrown ethers and an-thraquinone derivatives of triazole.
Alanso and coworkers reported a similar reaction,which used activated carbon-supported Cu nanoparticles.[65]This method offers low catalyst loading,shorter reaction times,and higher yields.Water was the best solvent for this procedure. Other azide precursors,including epoxides,diazonium salts, anilines,or alkenes,successfully gave the corresponding1,2,3-triazoles,[66,67]while the catalyst was reusable.
Recently,Chi et al.[29]established that Cu3N is an efficient cat-alyst for the Huisgen cycloaddition reaction(Table15).Cu3N nanoparticles are supported on a magnetic microsphere and are recyclable.As the catalyst is not cytotoxic,it can be applied to living and environmentally benign systems.It is expected to have considerable potential for use in a wide range of toxic-free click-chemistry applications,such as imaging and labeling of bio-molecules on living cells.The essential element for the activity of the catalyst is believed to be the Cu I species in Cu3N.The reaction is usually completed within3h at room temperature.
3.3.Carbonàoxygen bond formation
Diaryl ether is of considerable importance,as several com-pounds containing this moiety show significant biological ac-tivity.[68]Although the classical Ullmann coupling reaction is limited by elevated reaction temperatures,lower yields,and use of stoichiometric quantities of copper,is has been widely used in diaryl ether synthesis.[69]In the last decade,significant improvements have been made by using Cu nanoparticles as a catalyst.
3.3.1.Oxygen arylation
Cu II has been widely used in cross-coupling reactions of aryl halides and phenol to form a C(aryl)àO bonds.[70]A simple, general,and efficient procedure for the cross-coupling reaction of oxygen nucleophiles with aryl halides has been developed
1,3-Dipolar Huisgen cycloaddition reaction.
Time
[h]
2.5
8 [a]Reagents and reaction conditions:
alkyne(1mmol),sodium azide(1.1
1008C.nanoparticles as catalyst.
Product Yield
[%]
79
84 Reagents and reaction conditions:Alkyne(1mmol), (20mg),Et3N(0.3equiv.),acetonitrile(4mL);
Green Chemistry by Nanocatalysis
B.C.Ranu et al.
by using CuO nanoparticles under ligand-free conditions.[71]A variety of substrates underwent this reaction efficiently pro-ducing the corresponding biaryl ethers in excellent yields (Table 16).Dimethyl sulfoxide (DMSO)was the best solvent and KOH the most suitable base for C àO cross-coupling reactions.The reaction did not proceed in the absence of the catalyst.
Iodobenzene is more reactive compared to phenyl boronic acid,bromobenzene,and chlorobenzene.The substrates with electron-donating groups showed greater reactivity compared to those with electron-withdrawing groups.By using this pro-cedure,polyhydroxy compounds,pentaerythritol,and trietha-nolamine react with iodobenzene to provide the correspond-ing polyaryl ethers in excellent yields (Scheme 15).
As aryl iodides with electron-withdrawing groups have shown better reactivity compared to those with electron-do-nating groups,it was assumed that the reactions occurred through oxidative addition followed by a reductive elimination process.
Recently,Kantam et al.developed an efficient procedure for C àO coupling reactions using inexpensive and environmentally benign CuI nanoparticles as catalyst under ligand-free and rela-tively mild conditions.[72]A mixture of aryl chlorides,phenol,and K 2CO 3in dimethylformamide (DMF)gave excellent product yields when treated at 1208C under air (Table 17).
Both electron-donating substituents on aryl chlorides and electron-withdrawing substituents on phenols afforded the corresponding coupling products with uniform yields.Sterically hindered phenols retarded the reaction.It is believed that the reaction may occur through oxidative addition followed by re-ductive elimination.
3.3.2.Magnetic nanoparticle-catalyzed carbon àoxygen bond formation
The use of magnetic nanoparticles for C àO cross-coupling re-actions of phenol with aryl halides is very useful.Sun et al.[73]reported a Cu-Fe 2O 4nanoparticle-catalyzed diaryl ether forma-tion by using aryl halides,phenols,Cs 2CO 3as a base,and ace-tylacetone as a ligand in DMF at 1358C under argon (Scheme 16).The use of acetylacetone as a ligand appeared to be essential for the reaction.
Iodobenzenes with electron-rich substituents as well as those with electron-withdrawing groups reacted with a wide variety of phenols to give the desired products in good to ex-cellent yields.Sterically hindered aryl iodides were disfavored for this reaction.Substituents on the phenol ring influenced the reaction significantly.The catalyst could be reused six times with only a slight loss of activity for the reaction in each cycle.The catalyst was recovered by using an external magnet,washed with water and EtOAc,and dried under reduced pres-sure for use in the next cycle.
Halide Yield [%]Product
Halide I Br
8935
I
I Br 8832I Br [a]Reagents and reaction conditions:CuO NPs (2.5equiv),ROH (1aryl iodide (1.2equiv.),KOH (1.5equiv.),DMSO (1mL);1108
C.
Scheme 15.CuO nanoparticle-catalyzed reaction of pentaerythritol with aryl iodides.
O -Arylation of phenols with chlorobenzenes.Yield [%]Product
99
8798
[a]Reagents and reaction conditions:Phenol (1.2mmol),aryl (1.0mmol),catalyst (1.25mol %),K 2CO 3(1.2mmol),DMF
(1mL);5h.
Scheme 16.CuFe 2O4-catalyzed diaryl ether formation.Reagents:Aryl iodide (0.5mmol),phenol (1.0mmol).
G r e e n C h e m i s t r y b y N a n o c a t a l y s i s
Copper-Catalyzed Bond Formation with a Greener Perspective
3.3.3.Copper nanoparticle-catalyzed oxidative cyclization of Schiffs’base
Benz-fused azoles are an important class of compounds be-cause of their medicinal and biological activity.Kidwai et al.de-veloped a method for the synthesis of 2-arylbenzoxazoles through the condensation of aldehydes with 2-aminophenol using Cu nanoparticles under ligand-free conditions.[74]This one-pot synthesis includes in situ preparation of Schiff’s bases,which undergo oxidative cyclization in the absence of metal oxides,organic oxidizing agents,or strong acids.In this reac-tion,the subsequent formation of C àO and C àN bonds leads to the product (Table 18).
Under optimum conditions,the best yield was obtained for the reaction of 2-aminophenol with benzaldehyde in the pres-ence of 10mol %catalyst using K 2CO 3as a base.Methanol was the solvent of choice.The mechanism for the catalytic activity of the nanoparticles was highly dependent on the size of the Cu nanoparticles.
3.3.
4.Carbon àoxygen bond formation through Ullmann cou-pling reaction
Kidwai et al.[75]developed an efficient,economic,and novel method for the synthesis of diaryl ethers through Ullmann-type coupling reactions using recyclable Cu nanoparticles.This method provides wide substrate applicability,avoids the use of a heavy metal cocatalyst,and gives diaryl ethers in good yields (Table 19).
The best product yield was obtained with 50mol %Cu nanoparticles in 1.5h.Cs 2CO 3played an important role in the reaction because of its enhanced basicity.Substituted phenols with electron-donating groups provided better yields than those with electron-withdrawing groups.
3.3.5.Formation of aryl ethers and oxygen heterocycles using Cu 2O nanocubes
Park et al.[76]described a simple and economical process for the coupling reaction of aryl halides with phenols catalyzed by thermally and air stable Cu 2O nanocubes under ligand-free conditions.The process provides an easy access to a diverse range of diaryl ethers in high yields with low catalyst loading and in short reaction times (Scheme 17).
The relative reactivity towards a halophilic attack of phenol was investigated for R àCl,R àBr,R àI,and the order was found to be C àI >C àBr >C àCl.Although the electronic effect of the substituents in phenol was not prominent,substituents in aryl iodides influenced the yields of the reaction.Steric crowding of phenol also affected the rate of the reaction.
3.4.Carbon àsulfur bond formation
The formation of C àS bonds through transition metal-cata-lyzed cross-coupling reactions of aryl halides with sulfur nucle-ophiles is a powerful tool in organic synthesis.[77]The aryl sul-fides are useful intermediates in several organic transforma-tions and many of these compounds are pharmaceutically active.[78]The traditional method for the formation of C àS bonds often requires harsh conditions.To overcome these diffi-culties,attention has been focused on the development of cat-alytic system for C àS cross-coupling reactions.[79]
3.4.1.Carbon àsulfur cross-coupling reactions of thiols with io-dobenzene
Recently,the attention was focused on the development of catalytic systems for the C àS cross-coupling reactions of thiols with aryl halides.Punniyamurthy et al.[80]reported a highly effi-cient C àS cross-coupling reaction of aryl and alkyl thiols with
18.Synthesis of 2-arylbenzoxazoles.Yield [%]Product
9586[a]Reagents and reaction conditions:2-Aminophenol (1equiv.),hyde (1equiv.),K 2CO 3(2equiv.),Cu NPs (10mol %),MeOH;80–1000.1MPa,3–5h.
nanoparticles as catalyst.Yield [%]Product 85
68[a]Reagents and reaction conditions:Aryl iodide (1equiv.),(1equiv.),Cs 2CO 3(1.5equiv.),Cu NPs (10mol %),CH 3CN;50–60
0.1MPa.
Scheme 17.C àO coupling reactions catalyzed by Cu 2O nanocubes.
Green Chemistry by Nanocatalysis
B.C.Ranu et al.
iodobenzene using readily available,inexpensive,and air stable CuO nanoparticles.The authors screened several copper catalysts and found CuO nanoparticles to be the best catalyst.The reactions are effective at 808C in DMSO in the presence of KOH under nitrogen.Iodobenzene was more reactive than chlorobenzene or bromobenzene (Table 20).Substrates with electron-donating groups were more reactive than those with electron-withdrawing groups.
Thiols with a longer alkyl chain (octane-,decane-,and do-decanethiol)and benzyl thiol required longer reaction times compared to aryl thiols.This coupling reaction is suggested to proceed through oxidative addition followed by reductive elimination (Scheme 18).
3.4.2.Microwave-assisted ligand-free copper nanoparticle-cat-alyzed aryl àsulfur bond formation
Ranu et al.[20]demonstrated that the cross-coupling reaction of aryl halides with thiols was efficiently performed when using Cu 0nanoparticles and K 2CO 3under ligand-free conditions.This was the first report of C àS cross-coupling reactions using Cu 0nanoparticles.A variety of functionalized aryl sulfides were pre-pared in excellent yields under microwave irradiation for 5–7min (Table 21).
The electronic nature of the substituents on the aromatic rings of thiol and aryl iodide did not have a significant influ-ence on the reaction.Moreover,substitution at the ortho-posi-tion did not affect the reaction.The reaction was highly che-moselective with aryl iodide while keeping chloro and bromo groups intact.The catalyst was not very active after the first re-action and was not recyclable.
The probable mechanism involves the free-radical intermedi-ate generated by a one-electron transfer from Cu nanoparticles to thiols to form the RS radical,which interacts with ArI and produces Ar-S-R (Scheme 19)and releases an iodo radical for the further propagation of the cycle.
3.4.3.One-pot synthesis of S -aryl-and S -vinyl dithiocarba-mates using copper nanoparticles
Related to this type of C àS cross coupling reactions,Ranu et al.[81]introduced a convenient,green,and efficient proce-dure for the synthesis of aryl and vinyl dithiocarbamates by performing a simple one-pot three-component condensation of an amine,carbon disulfide,and an aryl iodide or a styrenyl bromide catalyzed by Cu nanoparticles in water.Significantly,they obtained exclusively (E )-and (Z )-products from the (E )-and (Z )styrenyl bromides,respectively.The reactions were highly stereoselective,the Cu nanoparticles were recyclable,and the reaction did not require a base (Table 22).
with iodobenzene.Yield [%]Product
90
98[a]Reagents and reaction conditions:CuO NPs (1.26mol %),thiol (1mmol),iodobenzene (1.1mmol),KOH (1.5mmol),DMSO 4–15h,
N 2.
Scheme 18.CuO nanoparticle-catalyzed C àS cross-coupling reactions of butane-1,4-dithiol with iodobenzene.Reagents:Aryl halide (iodide,bromide,chloride:1equiv.),phenol (1equiv.),Cu 2O nanocubes (0.1mol %),Cs 2CO 3(2equiv.).
nanoparticles.Yield [%]Product
91
7295
[a]Reagents and reaction Aryl iodide (1mmol),thiophenol (1.1mmol),Cu NPs (30(0.8mL),K 2CO 3(2mmol);120
7min.
Scheme 19.Probable mechanism of Cu nanoparticle-catalyzed aryl àsulfur bond formation.
G r e e n C h e m i s t r y b y N a n o c a t a l y s i s
Copper-Catalyzed Bond Formation with a Greener Perspective
The open chain as well as the cyclic amines participated in this reaction,and a wide range of aryl iodides and styrenyl bro-mides underwent reaction by this procedure.The mechanism involves oxidative addition of aryl or styrenyl halide to Cu nanoparticles to form ArCuI,which combines with the dithio-carbamate anion,which is generated in situ by the reaction of the amine and carbon disulfide,to give an intermediate A ,which yields the product by subsequent reductive elimination
(Scheme 20).The liberated Cu 0initiates further reaction and propagates the cycle.Cu 0nanoparticles facilitated the oxida-tive coupling reaction with an aryl iodide by an easier transfer of an electron in comparison to metallic Cu.Water also played an important role in this reaction.
3.4.4.Stereoselective synthesis of vinyl sulfides catalyzed by using copper(II)oxide nanoparticles
Rao and coworkers [82]developed a method for the cross-cou-pling reaction of vinyl halides with thiols catalyzed by CuO nanoparticles under ligand-free conditions.During optimiza-tion,KOH was found to be the ideal base for the reaction.With increasing temperature,the yield increased and reached an optimum at 808C.Among the various solvents and catalysts tested,combination of nano-CuO with KOH in DMSO provided the best result (Table 23).
In the absence of metal oxide nanoparticles,the reaction did not produce any C àS cross-coupling products.The yields were highly dependent on the reaction temperature,base,solvent,and the nature of catalyst.This protocol works uniformly for
trans -b -iodostyrene with electron-rich,electron-neutral,and electron-deficient groups to produce the corresponding prod-ucts in excellent yields.For vinyl bromide,a longer reaction time was needed to obtain a good yield.The catalyst was easily recovered and reused for four cycles without significant loss of activity.
3.4.5.Synthesis of aryl sulfides through cascade reaction of aryl halides with thiourea
Nageswar et al.[83]reported a recyclable CuO nanoparticle-cata-lyzed synthesis of symmetrical diaryl sulfides under ligand-free conditions in the absence of any additive.The reaction used a simple catalyst system,which provided a broad functional group tolerance and good to excellent yields.The reaction was effective when using polar protic solvents,such as DMSO and DMF.Among the various bases (KOH,Cs 2CO 3,Na 2CO 3,NaOH)investigated,Cs 2CO 3was the most effective.The optimum temperature for the reaction was 1108C (Table 24).
with dithiocarbamate anion.Yield [%]
Product
8087[a]Reagents and reaction conditions:Aryl iodide/styrenyl bromide (1mmol),Cu NPs (3mol %),carbon disulfide (2.5mmol),(1.2
mmol),water;reflux,6h.
Scheme 20.Probable mechanism for the formation of aryl/vinyl thio-carbamate.
23.Cross-coupling reaction of thiol with trans -b -iodostyrene.Product
Yield [%]95
93
Reagents and reaction conditions:Trans -b -iodostyrene mmol),CuO NPs (1.5mmol),KOH Green Chemistry by Nanocatalysis
B.C.Ranu et al.
Iodobenzenes with electron-donating groups(e.g.,Me,Et, and OMe)produce the corresponding aryl sulfides in excellent yields,whereas those with electron-withdrawing groups(NO2)
show a decrease in the yield of the diaryl sulfide.A longer reaction time is needed for aryl bromides and hetroaromatic bromides.
3.5.Carbonàselenium bond formation
Diaryl selenides play an important role in organic chemistry, acting as versatile reagents in organic synthesis[84]and cataly-sis.[85]The biological and medicinal properties of selenium and organoselenium compounds are also increasingly appreciated as anticancer and antioxidant agents.[86]Cu-catalyzed reac-tions[87]of AràSeàAr have been of considerable interest,possi-bly because of the easy availability and low cost of copper derivatives and easy handling of the selenium reagent.
3.5.1.Coupling reaction of diaryl diselenide and aryl halides under ligand-free conditions
A new,efficient,and ligand-free cross-coupling reaction of aryl halides and diaryl diselenides using catalytic amounts of nano-crystalline CuO as a catalyst and with KOH as the base in DMSO at1108C was reported by Rao et al.[88]This procedure was utilized for the synthesis of a variety of aryl selenides in excellent yields from the readily available aryl halides and diaryl diselenides.In a preliminary experiment,when iodoben-zene was treated with diphenyl diselenide in the presence of 2mol%of CuO nanoparticles and KOH(2.0equiv)in DMSO (2.0mL)for12h,the corresponding diaryl selenide was ob-tained in94%yield(Table25).This is the first CuO nanoparti-cle-catalyzed coupling reaction of aryl halides with diphenyl diselenide to form diaryl selenide.
In general,all reactions are clean,and the diaryl selenides are obtained in high yields.This procedure is compatible with diphenyl diselenide with electron-rich,electron-deficient,and sterically crowded ortho-substituted aryl iodides.KOH and Cs2CO3worked best with this system and gave moderate to ex-cellent yields compared to K3PO4,KO t Bu,and NaOMe.Solvents other than DMSO,such as DMF and toluene,were less effec-tive.The effect of different combinations of various metal oxide nanoparticles on the diaryl selenide formation with KOH in DMSO at1108C using different substituted aryl iodides was investigated.However,their catalytic activities were considera-bly lower than that of CuO nanoparticles.The yields were highly dependent on the reaction temperature and the base.
3.5.2.Synthesis of symmetrical diaryl selenides using sele-nourea and aryl halides/boronic acids
Selenourea is used as an effective selenium surrogate in the CàSe cross-coupling reaction catalyzed by CuO nanoparticles under ligand-free conditions.Rao et al.[89]reported the synthe-sis of a variety of symmetrical diaryl selenides in good to excel-lent yields by the reaction of aryl halides/boronic acids and se-lenourea.The best result was obtained when the reaction was performed at808C using3.0mol%of the CuO nanoparticles in the presence of KOH(2.0equiv)and DMSO(2.0mL,Table26). The catalyst maintained its good level of activity even after being recycled four times.
Iodobenzenes with electron-donating groups(e.g.,Me,Et, and OMe)reacting with selenourea produced the correspond-ing selenides in excellent yields,but for electron-withdrawing groups a decrease in the yield of the diaryl selenide was ob-served.Iodobenzene was more reactive than bromo-and chlorobenzenes,thus requiring shorter reaction times.This protocol was also applied to the cross-coupling reaction of aryl boronic acids with selenourea,and the corresponding diaryl selenides were obtained in high yields with the same catalyst. This CuO nanoparticle-catalyzed CàSe cross-coupling reaction
24.CuO nanoparticle-catalyzed synthesis of aryl sulfides.
Yield
[%]
Product
87
90
75
[a]Reagents and reaction conditions:Aryl iodide(2mmol),
(1.2mmol),CuO NPs(5mol CO3(2equiv.),DMSO(2mL); 15h.CuO nanoparticle-catalyzed CàSe coupling reaction.
Product Yield
[%]
85
89
82
and reaction conditions:Aryl
equiv.),CuO NPs(2equiv.),
h,N2atmosphere.
G
r
e
e
n
C
h
e
m
i
s
t
r
y
b
y
N
a
n
o
c
a
t
a
l
y
s
i
s
Copper-Catalyzed Bond Formation with a Greener Perspective
of aryl halides and selenourea proceeds through the oxidative addition of aryl halide to the CuO nanoparticles leading to the formation of I ,which undergoes reaction with selenourea to give intermediate II .This intermediate provides III through re-ductive elimination,which produces a benzeneselenate moiety and urea upon hydrolysis in the reaction mixture (Scheme 21).In this step,urea is hydrolyzed in situ to produce carbon diox-ide and ammonia.Oxidative addition of aryl iodide to CuO nanoparticles leads to the formation of I ,which undergoes re-action with benzeneselenate anion to give intermediate IV ,which produces diaryl selenide through reductive elimination.
3.5.3.Cross-coupling reactions of organic diselenide with aryl boronic acid
CuO nanoparticle-catalyzed coupling reactions of organic dise-lenides with aryl boronic acids to form organic selenides has been reported by Alves et al.[90]This ligand-free coupling reac-tion involved the use of CuO nanoparticles (3mol %),diphenyl diselenide (0.25mmol),phenyl boronic acid (1.5equiv),and DMSO as a solvent.The heterogeneous reaction mixture was
stirred for 24h at 1008C under air to afford the corresponding diaryl selenide in high yields (Scheme 22).
The reaction is not sensitive to electronic effects of the sub-stituents attached to the aromatic ring of the boronic acid,al-though the nature of substitution in the aromatic ring of the diaryl diselenide has a considerable influence on the reaction.Aryl boronic acids bearing both electron-donating and elec-tron-withdrawing groups gave uniform yields of the product.However,the diaryl diselenides bearing electron-donating groups gave lower yields than those with electron withdraw-ing groups.A decrease in the yield of the product was also ob-served in the coupling reaction of hindered boronic acid.
3.5.
4.Phenyl-selenylation of aryl iodides and vinyl bromides in water
Ranu et al.[21]developed a green and efficient method for the coupling reaction of aryl iodides/vinyl bromide with diphenyl diselenides in the presence of Cu 0nanoparticles and zinc in water.The reaction proceeds well in water under reflux giving the best yield when using 20mol %of Cu 0nanoparticles.With a lesser amount of Cu 0nanoparticles,the reaction remained in-complete within a reasonable time https://www.360docs.net/doc/1011790454.html,bination of zinc with Cu 0nanoparticles is essential for this coupling reac-tion (Table 27).
The reaction proceeded without any difficulty with both electron-donating and electron-withdrawing substituents on the aromatic ring of the aryl iodides.The reactions with (E )-styrenyl bromides were highly stereoselective giving (E )-sele-nides.However,the (Z )-vinyl bromides produced mixtures of (E )-and (Z )-isomers.A change of the solvent from water to DMF or THF did not improve the stereoselective distribution of the product.The aliphatic vinyl bromide also participated in this reaction.Regarding the mechanism of this reaction,it was suggested that Cu 0nanoparticles readily undergo oxidative ad-dition with diphenyl diselenide to form the intermediate (PhSe)2Cu II .Upon reduction by zinc,this intermediate leads to the key intermediate PhSeCu I ,which reacts with ArI to give the product ArSePh via a transient Cu III intermediate (Scheme 23).The CuI generated in the reaction of PhSeCu I with ArI is re-duced by zinc to regenerate Cu 0nanoparticles,which initiate the next cycle.The Zn(SePh)2formed in the reduction process of Cu(SePh)2with zinc reacts with CuI to give PhSeCu I ,which undergoes further reaction.Thus,both PhSe moieties of PhSeSePh are used up in the process.
26.Cu-catalyzed synthesis of symmetrical diaryl selenides.Product
Yield [%]Product
91
86[a]Reagents and reaction Aryl iodide (2equiv.),selenourea (1equiv.),CuO NPs (3equiv.),equiv),
DMSO (2mL);10020
h.
Scheme 21.Probable mechanism for the selenylation reaction.
Scheme 22.Cross-coupling reactions of organic diselenides and aryl boronic acids catalyzed by CuO nanoparticles.Reagents and reaction conditions:Diphenyl diselenide (0.5mmol),aryl boronic acid (1.5equiv.),DMSO (1mL).
Green Chemistry by Nanocatalysis
B.C.Ranu et al.
3.6.Miscellaneous
3.6.1.Reduction of the nitro group
Recently,Ranu et al.[91]demonstrated that Cu nanoparticles in combination with ammonium formate offered chemoselective reduction of aromatic nitro compounds to the corresponding amino derivatives (Table 28)at 1208C in EG.Although the re-duction of the nitro to the amino group is a classical reaction and can be performed by using a variety of reducing agents,it was often observed that selective reduction of the nitro group in the presence of other reducible functional groups is not sat-isfactory.Thus,an alternative efficient,simple,and chemoselec-tive procedure developed by these authors is of considerable significance.
The reductions are successfully performed in the presence of a wide variety of other reducible functional groups in the molecules,such as àCl,àI,àOCH 2Ph,àNHCH 2Ph,àCOR,àCOOR,or àCN.To perform the reduction,Cu nanoparticles and ammonium formate are complementary to each other.5equiv.
of ammonium formate are required for complete conversion,and this might be attributable to the fact that a portion of the ammonium formate,deposited by sublimation on the wall of the condenser with the progress of the reaction,remained un-available for the reaction,thus requiring a higher than stoichio-metric amount.The reactions are clean and high yielding.
The cost effectiveness of Cu nanoparticles compared to other noble metals,such as Au [92]and Pt [93]nanoparticles re-ported earlier for this reduction,make it more useful for indus-trial purpose.
3.6.2.Hydrogenolysis of glycerol
As glycerol is a main byproduct in the production of biodiesel obtained through transesterification of vegetable oils and animal fats,large quantities of glycerol are available.[94]Thus,the appropriate utilization of glycerol would be appreciated.One of the attractive uses of glycerol is to produce glycols,es-pecially propanediols,by using an alternative route,which in-volves selective hydrogenolysis of glycerol.Recently,Chen and Xia et al.[26]developed silica-supported Cu nanoparticles as effi-cient catalysts for glycerol hydrogenolysis (Scheme 24).This process provides a clean and economically competitive route for the production of these useful chemicals from glycerol.
3.6.3.Three-component coupling reactions
The three-component coupling reactions of thiazolidine-2,4-dione (TZD),aromatic aldehyde,and ammonium acetate (Scheme 25)using Cu nanoparticles as catalyst in an ionic liquid at room temperature was reported by Chandra et al.[95]
Yield [%]Product
87
8886[c]
[a]Reagents and reaction conditions:Styryl bromide/aryl iodide diphenyl diselenides (0.5equiv.),Zn dust (1.5equiv.)Cu NPs H 2O;reflux,10–12h.[b]E /Z =20:80.[c]E /Z =
32:68.
Scheme 23.Probable mechanism for the selenylation reaction.
nanoparticle-catalyzed chemoselective nitroreduction.Yield [%]Product 83
8675[a]Reagents and reaction conditions:Nitroarenes (1mmol),(3mmol),HCOONH 4(5mmol),ethylene glycol (10mL);120
8C,Scheme 24.Cu nanoparticle-catalyzed hydrogenolysis of glycerol.Reagents and reaction conditions:Aqueous solution of glycerol (80g of 80wt %),cat-alyst (4g),H 2pressure 9.0MPa;12h.
G r e e n C h e m i s t r y b y N a n o c a t a l y s i s
Copper-Catalyzed Bond Formation with a Greener Perspective
个人申请新能源汽车充电桩小区安装流程完整版
个人申请新能源汽车充电桩小区安装流程 标准化管理处编码[BBX968T-XBB8968-NNJ668-MM9N]
个人申请新能源汽车充电桩安装流程 2017-10-20 21:13:51 广顺安 5425 随着电动汽车技术越来越成熟,大城市汽车限购政策越来越严格,电动汽车逐渐走进我们的生活。按照目前的电动汽车政策,购买电动汽车可以送充电桩,充电桩的安装流程是什么呢? 第一步:联系物业公司,确定小区充电桩安装政策。 目前对于安装充电桩,一般的规定是要求自有产权的车位。另外,有些物业由于建设比较早,不太适宜安装充电桩,如果安装充电桩需要额外的技术设备投入,一定要和物业提前沟通好。 第二步:考察自己的车位。 这种考察是指业主自己去实地考察,这个步骤对于节省成本来说非常重要。一般小区都会有很多配电室,业主要自己选择一个距离车位最近的,这样在安装的时候可以省很多费用。 第三步:提供相关材料。 将身份证复印件、车位产权证明(可以由物业出具)、物业同意安装说明交给工作人员,目前北京市的电力公司只接受充电桩公司的申请,业主是不能自己去申请的。 第四步:实地考察。 电力公司的工程师会通知充电桩公司一个时间,届时物业电工、充电桩公司、电力公司和业主都要在现场,确定最终施工方案。 第五步:安装。 确定施工方案后,就到了安装施工的步骤,根据各小区条件和车库位置的不同,施工时间也不同,有的只需要2小时就搞定,有的可能需要一整天才能施工完成。 第六步:验收。 施工完成后由充电桩公司会去电力公司报备,由电力公司去现场验收,验收合格会给电表施加封志,然后电力公司会制作对应的电卡,并由充电桩公司领取转交业主,或者业主自己去供电具领取。 如有需要可以联系我叶
新能源汽车充电桩投资项目可行性分析
新能源汽车充电桩投资项目 可行性分析 规划设计 / 投资分析
摘要 该新能源汽车充电桩项目计划总投资4516.85万元,其中:固定资产 投资3857.55万元,占项目总投资的85.40%;流动资金659.30万元,占项目总投资的14.60%。 达产年营业收入4446.00万元,总成本费用3339.24万元,税金及附 加73.07万元,利润总额1106.76万元,利税总额1332.49万元,税后净 利润830.07万元,达产年纳税总额502.42万元;达产年投资利润率 24.50%,投资利税率29.50%,投资回报率18.38%,全部投资回收期6.94年,提供就业职位72个。 严格遵守国家产业发展政策和地方产业发展规划的原则。项目一定要 遵循国家有关相关产业政策,深入进行市场调查,紧密跟踪项目产品市场 走势,确保项目具有良好的经济效益和发展前景。项目建设必须依法遵循 国家的各项政策、法规和法令,必须完全符合国家产业发展政策、相关行 业投资方向及发展规划的具体要求。 总论、项目基本情况、产业分析、项目建设内容分析、项目选址分析、土建工程分析、工艺概述、项目环境影响情况说明、生产安全保护、项目 风险、节能评价、项目计划安排、投资计划方案、项目经营收益分析、结 论等。
新能源汽车充电桩投资项目可行性分析目录 第一章总论 第二章项目基本情况 第三章产业分析 第四章项目建设内容分析 第五章项目选址分析 第六章土建工程分析 第七章工艺概述 第八章项目环境影响情况说明 第九章生产安全保护 第十章项目风险 第十一章节能评价 第十二章项目计划安排 第十三章投资计划方案 第十四章项目经营收益分析 第十五章项目招投标方案 第十六章结论
智能车辆安全辅助驾驶技术研究近况
文章编号:1002O0268 (2007)07O0107O05 智能车辆安全辅助驾驶技术研究近况 基金项目: 国家自然科学基金资助项目 () 作者简介: 王荣本(1946-),男,教授,博士生导师, 研究方向为智能车辆、汽车安全辅助驾驶、物流自动化 xx,xx,xx,xx,余天xx (吉林大学交通学院,吉林长春130025) 摘要: 论述了安全辅助驾驶技术的研究现状、研究的必要性以及研究进展。安全辅助驾驶技术包括车道偏离预警与保持、前方车辆探测及安全车距保持、行人检测、驾驶员行为监测、车辆运动控制与通讯等。分析了各种传感器的优缺点及其在实际应用过程中存在的问题,基于单一传感器不能很好地解决安全辅助驾驶技术可靠性和环境适应能力的要求,应结合激光雷达技术解决图像模糊问题,利用红外传感器增强机器视觉识别的可靠性,未来的安全辅助驾驶技术应该采取多种传感器融合的技术,结合毫米波雷达和激光雷达系统具有深度测量精确的特点,将极大的推动汽车安全辅助驾驶系统的应用和推广。 关键词: 智能交通系统;安全辅助驾驶;车道偏离预警;行人检测;车间通讯中图分类 号:
U491文献标识 码:AReviewontheResearchofIntelligentVehicleSafetyDrivingAssistantTechnology WANGRongOben,GUOLie,JINLiOsheng,GUBaiOyuan,YUTianOhong (SchoolofTransportation,JilinUniversity,Jilin Changchun 130025,China) Abstract: Keywords: 引言 智能车辆是利用传感器技术、信号处理技术、通讯技术、计算机技术等,辨识车辆所处的环境和状态,根据各传感器所得到的信息做出分析和判断,或者给司机发出劝告和报警信息,提请司机注意规避危险;并能在紧急情况下,帮助司机操作车辆(即辅助驾驶),防止事故的发生。 早期智能车辆研究主要集中在如何采用各种传感器技术实现车辆全自动化无人驾驶,随着研究的深入,重点着眼于提高汽车的安全性、舒适性以及提供优良的人车交互界面,并努力向市场推广智能车辆相关技术的应用。 1998年美国运输部认为日益严重的交通事故是最迫切需要解决的问题,开始组织实施智能车辆先导IVI(IntelligentVehicleInitiative)计划。该计划的基本宗旨和目标是预防交通事故及其引起的人员伤亡,提高安全性,并以人为因素为基础,防止驾驶员精神分散,促进防撞系统的推广应用。 智能车辆技术研究重点的转移主要是日渐增长的交通事故以及对减少驾驶员操作强度的需求。根据美国运输部IVI计划,仅在美国,每年至少发生680万起交通事故,造成412万人死亡。 在一些发达国家,情况就更严重。如我国在2004年共发生道路交通事故517889起,造成1077人死亡,直接财产损失2319亿元,与2003年相比,死亡人数上升216%。1安全辅助驾驶技术的研究现状 安全辅助驾驶技术主要目的是提高汽车行驶的安全性,通过安装在车辆及道路上的各种传感器掌握本车、道路以及周围车辆的状况等信息,为驾驶员提供劝
深圳大厦充电桩建设方案
深圳大厦充电桩建设方 案 文稿归稿存档编号:[KKUY-KKIO69-OTM243-OLUI129-G00I-FDQS58-
深圳****大厦充电桩建设方案一、充电桩建设的目的和意义 世界能源需求的不断攀升,环境污染越来越严重,汽车作为主要的交通工具,也是城市的主要污染源。电动汽车产业在新能源背景下蓄势勃发,已经成为流行最广、节能环保的绿色出行交通工具。 新能源汽车作为新的产业,整个产业还处于发展初期。现在整个行业都面临着有充电桩没有电动汽车充电或有电动汽车没有充电桩的尴尬局面。在新能源汽车发展的初期,建设越多的充电桩意味着亏损越多,导致新能源汽车充电设施的建设非常缓慢。 为了加快新能源汽车产业的发展,国家和各地方出台了房地产项目中充电桩建设的强制措施,这样就出现前期投入建设充电桩较多,但是充电的新能源汽车比较少,投资收益比非常不理想的情况。 随着电动汽车的逐渐推广,公共充电站数量也会随国家政策导向逐年增加,当写字楼或大型商圈周围遍布充电设备时,运营商之间竞争会更加激烈,为吸引客户前来充电、入驻大厦,减免充电服务费甚至免费充电会成为一种充电桩运营的趋势。 在投资收益比不理想甚至将来面临零收益的情况下,如何维持充电桩运营、实现整体性的盈利就成了一个急需解决的问题。**公司提供充电设备为附带32寸液晶显示屏充电桩,在解决为新能源汽车提供充电服务的同时,还可投放充电桩使用方法、注意事项、安全须知、停车指示、服务推送以及符合业主及物业品牌要求的商业广告等内容。通过广告运营可以获得较大收益,从而大幅降低充电桩运营成本,实现盈利。 二、充电桩设计方案 2.1设计原则 根据现场勘查,项目停车位数量:140个,需要配置6台充电桩,暂时确定充电桩数量(6台);结合广告受众人群流量大小,广告桩安装位置优先选择靠近人
新能源汽车充电桩使用管理规定精编版
新能源汽车充电桩使用管理规定 一、操作人员管理要求 1、充电桩操作人员必须经国家有关部门培训考核合格并持有颁发的资质证后上岗,同时需接受安全教育和岗位技能培训。操作人员应佩戴或在场站内的醒目位置悬挂标明个人姓名、工号、岗位的标志。 2、充电作业时须穿戴专业绝缘防护鞋及绝缘防护手套,保持自身、车体、充电桩及周边区域干燥。 3、操作人员应主动引导车辆进入充电位置,当车辆停稳,切断电动汽车动力电源和辅助电源,拉紧手刹,人员离车后,方可进行充电作业。驾驶员自觉服从站点工作人员的安排。 4、充电前,操作人员应检查充电接口是否正常完好,并对车辆进行充电前检查,对充电设备与电动汽车连接和充电参数的设置进行确认。 5、充电启动后,确认充电正常,并定期巡视充电状态。发生安全事故,应快速按下红色急停按钮,切断电源。 6、充电过程中,车辆严禁启动或移动,严禁带电插拔充电插头。充电结束后、行车前,驾驶员应确认充电终止以及充电设备与电动汽车物理分离。 7、严禁使用金属物体触碰充电枪接口、纯电车充电口。 8、操作人员应基本了解电动汽车的构造和充电设备的工作原理,了解动力蓄电池应用的基础知识,掌握充电操作规程、充电设备检测、
故障判断和处理、安全知识和应急处理方法。 9、操作人员应按照充电桩生产厂家的顾客手册进行定期保养与例行检查,保持其安全、清洁、完好,并做好相关检查保养记录。 10、充电站每日应做好站内日查,当班管理人员应对作业现场进行监督,发现违章行为和不安全因素,有权制止并向上级反映情况。充电作业人员应定期或根据工作需要随时进行巡视。 二、充电桩使用和管理 1、充电人员必须定期检查充电桩及其他相关设备,消防器材、设施设备保持清洁干燥,并做相关检查记录,按要求上报。定时对充电场地、充电设备设施、消防器材保洁,确保设备情况良好。 2、充电过程中,操作人员应按照操作流程操作,如厂家有其他充电要求,则按照厂家要求进行操作。同时须按要求对充电桩仪表、数据、充电模块、线路、开关等设施进行检查,并按要求填写巡检记录。 3、充电过程中如发生故障,充电人员应立即按下充电机上的急停按键,以防故障进一步扩大,并上报技术科,由专业人员进行设备维修。 4、如遇系统起火时,首先动用紧急停机装置切断电源,然后使用ABC通用型灭火器或者二氧化碳灭火器灭火,严禁使用泡沫灭火器和水灭火。 5、充电结束后,应按规定拔除充电枪,将线缆理好放在线架上,
新能源汽车充电桩使用管理规定解析
新能源汽车充电桩使用管理规定解析新能源汽车充电桩使用管理规定 一、操作人员管理要求 1、充电桩操作人员必须经国家有关部门培训考核合格并持有颁发的资质证后上岗,同时需接受安全教育和岗位技能培训。操作人员应佩戴或在场站内的醒目位置悬挂标明个人姓名、工号、岗位的标志。 2、充电作业时须穿戴专业绝缘防护鞋及绝缘防护手套,保持自身、车体、充电桩及周边区域干燥。 3、操作人员应主动引导车辆进入充电位置,当车辆停稳,切断电动汽车动力电源和辅助电源,拉紧手刹,人员离车后,方可进行充电作业。驾驶员自觉服从站点工作人员的安排。 4、充电前,操作人员应检查充电接口是否正常完好,并对车辆进行充电前检查,对充电设备与电动汽车连接和充电参数的设置进行确认。 5、充电启动后,确认充电正常,并定期巡视充电状态。发生安全事故,应快速按下红色急停按钮,切断电源。 6、充电过程中,车辆严禁启动或移动,严禁带电插拔充电插头。充电结束后、行车前,驾驶员应确认充电终止以及充电设备与电动汽车物理分离。 7、严禁使用金属物体触碰充电枪接口、纯电车充电口。 8、操作人员应基本了解电动汽车的构造和充电设备的工作原理,了解动力蓄电池应用的基础知识,掌握充电操作规程、充电设备检测、 故障判断和处理、安全知识和应急处理方法。 9、操作人员应按照充电桩生产厂家的顾客手册进行定期保养与例行检查,保持其安全、清洁、完好,并做好相关检查保养记录。
10、充电站每日应做好站内日查,当班管理人员应对作业现场进行监督,发现违章行为和不安全因素,有权制止并向上级反映情况。充电作业人员应定期或根据工作需要随时进行巡视。 二、充电桩使用和管理 1、充电人员必须定期检查充电桩及其他相关设备,消防器材、设施设备保持清洁干燥,并做相关检查记录,按要求上报。定时对充电场地、充电设备设施、消防器材保洁,确保设备情况良好。 2、充电过程中,操作人员应按照操作流程操作,如厂家有其他充电要求,则按照厂家要求进行操作。同时须按要求对充电桩仪表、数据、充电模块、线路、开关等设施进行检查,并按要求填写巡检记录。 3、充电过程中如发生故障,充电人员应立即按下充电机上的急停按键,以防故障进一步扩大,并上报技术科,由专业人员进行设备维修。 4、如遇系统起火时,首先动用紧急停机装置切断电源,然后使用ABC通用型灭火器或者二氧化碳灭火器灭火,严禁使用泡沫灭火器和水灭火。 5、充电结束后,应按规定拔除充电枪,将线缆理好放在线架上, 锁好充电口及车门,并记录相关数据。 6、各类台账记录完善,分类明细准确。 7、严禁私自拆卸、改装充电桩设备及附加设施,对因此造成的损坏由当事人承担相应的经济责任,并对管理责任人进行问责。 三、充电场站管理 1、充电站所属公司应设置安全管理组织机构,并配备专职或兼职的安全员,要建立安全管理制度和应急预案,建设安全应急保障体系。并负责制、修订充电站管理责任书,负责指导、监督、评估充电站安全管理工作。
新能源汽车充电桩项目初步方案
新能源汽车充电桩项目 初步方案 规划设计/投资分析/实施方案
报告说明— 该新能源汽车充电桩项目计划总投资10798.99万元,其中:固定资产 投资7982.40万元,占项目总投资的73.92%;流动资金2816.59万元,占 项目总投资的26.08%。 达产年营业收入26791.00万元,总成本费用20662.15万元,税金及 附加232.31万元,利润总额6128.85万元,利税总额7206.52万元,税后 净利润4596.64万元,达产年纳税总额2609.88万元;达产年投资利润率56.75%,投资利税率66.73%,投资回报率42.57%,全部投资回收期3.85年,提供就业职位541个。 作为新能源汽车可持续发展的关键因素之一,充电设施建设和完善备 受关注。中国电动汽车充电基础设施促进联盟发布的截至2019年4月的数 据显示,中国充电基础设施仍保持高速发展态势。目前全国充电基础设施 数量达到95.3万台,是全球规模最大的充电设施市场。从竞争格局来看, 北上广保有量位居前列,特来电运营设施数量最多。虽然我国充电基础设 施规模居世界首位,但充电设施的建设和管理运营仍存在不少问题。目前,各运营商充电设施利用率普遍低于15%,我国充电设施产业面临充电需求难以满足和设施利用率低的双重压力。
第一章总论 一、项目概况 (一)项目名称及背景 新能源汽车充电桩项目 (二)项目选址 xxx高新区 项目建设方案力求在满足项目产品生产工艺、消防安全、环境保护卫生等要求的前提下尽量合并建筑;充分利用自然空间,坚决贯彻执行“十分珍惜和合理利用土地”的基本国策,因地制宜合理布置。投资项目对其生产工艺流程、设施布置等都有较为严格的标准化要求,为了更好地发挥其经济效益并综合考虑环境等多方面的因素,根据项目选址的一般原则和项目建设地的实际情况,该项目选址应遵循以下基本原则的要求。 (三)项目用地规模 项目总用地面积32716.35平方米(折合约49.05亩)。 (四)项目用地控制指标 该工程规划建筑系数54.28%,建筑容积率1.08,建设区域绿化覆盖率7.45%,固定资产投资强度162.74万元/亩。
发动机燃料供给系统
第二节发动机燃料供给系统 一、燃料供给系统功能及结构概述 燃料供给系统(供油系统)的功能:对发动机的性能而言,燃料系统主要具有将不含有灰尘、水分和空气等杂质的干净燃料输送给发动机的功用。此系统与发动机的输出功率、排气烟度以及高压油泵、喷油器的正常工作等发动机故障现象也有着密切的关联。柴油机燃料供给系统的任务,是根据柴油机工作的需要,定时、定量、定压地将柴油按一定的供油规律成雾状喷入燃烧室内与空气迅速混合燃烧。 柴油机燃料供给系统由下列组成: 1.燃油系统工作流程图(图1-2-1) 图1-2-1 燃油系统工作流程图
燃油供给装置包括:燃油箱总成、燃油粗滤器、输油泵、进油管、燃油精滤器、高低压油管、喷油器和回油管。燃油供给装置的功能在于贮存、输送、清洁,提高柴油压力,通过喷油嘴呈物状喷入燃烧室与空气混合而成可燃混合气。 二、燃油供给系统的主要零部件 有关输油泵、燃油滤清器、调速器、角度自动提前器、喷油泵、喷油器的结构、原理、修理、保养请参看该发动机的使用维护说明书。1.带锁燃油箱总成(图1-2-2) 该车型的带锁燃油箱总成按容积共分3个系列,容量分别为400L、320L、270L。一般情况燃油箱总成放置在汽车前进方向的右侧,空滤总成的后部。该燃油箱总成采用钢板卷压成型,端盖咬接答焊,内表面防腐密封处理。具有耐腐蚀、防锈和不易泄漏,容积大等优点。 油箱的中上部是加油口,加油口直径为φ100mm,加油口高出燃油箱45mm,为了加油方便,加油管内带有可以拉出的延伸管,延伸管底部装有铜丝滤网。油箱盖由耐油橡胶垫密封,靠三爪弹簧片锁紧,在油箱盖上并设有通气孔,排出油箱内的蒸汽,保持内外气压一致。油箱盖上装有链索扣环,与加油管内的延伸管相连,以免盖子失落。
新能源汽车充电桩项目规划方案
新能源汽车充电桩项目 规划方案 投资分析/实施方案
承诺书 申请人郑重承诺如下: “新能源汽车充电桩项目”已按国家法律和政策的要求办理相关手续,报告内容及附件资料准确、真实、有效,不存在虚假申请、分拆、重复申请获得其他财政资金支持的情况。如有弄虚作假、隐瞒真实情况的行为,将愿意承担相关法律法规的处罚以及由此导致的所有后果。 公司法人代表签字: xxx有限公司(盖章) xxx年xx月xx日
项目概要 作为新能源汽车可持续发展的关键因素之一,充电设施建设和完善备 受关注。中国电动汽车充电基础设施促进联盟发布的截至2019年4月的数 据显示,中国充电基础设施仍保持高速发展态势。目前全国充电基础设施 数量达到95.3万台,是全球规模最大的充电设施市场。从竞争格局来看, 北上广保有量位居前列,特来电运营设施数量最多。虽然我国充电基础设 施规模居世界首位,但充电设施的建设和管理运营仍存在不少问题。目前,各运营商充电设施利用率普遍低于15%,我国充电设施产业面临充电需求难以满足和设施利用率低的双重压力。 该新能源汽车充电桩项目计划总投资6162.09万元,其中:固定 资产投资4933.44万元,占项目总投资的80.06%;流动资金1228.65 万元,占项目总投资的19.94%。 达产年营业收入8738.00万元,总成本费用6815.82万元,税金 及附加103.85万元,利润总额1922.18万元,利税总额2291.16万元,税后净利润1441.63万元,达产年纳税总额849.53万元;达产年投资 利润率31.19%,投资利税率37.18%,投资回报率23.40%,全部投资回收期5.77年,提供就业职位179个。 坚持应用先进技术的原则。根据项目承办单位和项目建设地的实 际情况,合理制定项目产品方案及工艺路线,在项目产品生产技术设
(完整版)高级驾驶辅助系统ADAS各功能详解
ADAS(高级驾驶辅助系统)高级驾驶辅助系统(Advanced Driver Assistant System),简称ADAS,是利用安装于车上的各式各样的传感器,在第一时间收集车内外的环境数据,进行静、动态物体的辨识、侦测与追踪等技术上的处理,从而能够让驾驶者在最快的时间察觉可能发生的危险,以引起注意和提高安全性的主动安全技术。ADAS 采用的传感器主要有摄像头、雷达、激光和超声波等,可以探测光、热、压力或其它用于监测汽车状态的变量,通常位于车辆的前后保险杠、侧视镜、驾驶杆内部或者挡风玻璃上。早期的ADAS 技术主要以被动式报警为主,当车辆检测到潜在危险时,会发出警报提醒驾车者注意异常的车辆或道路情况。对于最新的ADAS 技术来说,主动式干预也很常见。ADAS通常包括以下17种用与汽车驾驶辅助的系统: 1、导航:导航是一个研究领域,重点是监测和控制工艺或车辆从一个地方移动到另一个地方的过程。导航领域包括四个一般类别:陆地导航,海洋导航,航空导航和空间导航。 2、时交通系统TMC:TMC是是欧洲的辅助GPS导航的功能系统。它是通过RDS方式发送实时交通信息和天气状况的一种开放式数据应用。借助于具有TMC功能的导航系统,数据信息可以被接收并解码,然后以用户语言或可视化的方式将和当前旅行路线相关的信息展现给。 3、电子警察系统ISA:我国道路交通管理系统中的“电子警察”是随着科技的发展而产生的,是一个时代的产物。它作为现代道路交通安全管理的有效手段,可以迅速地监控、抓拍、处理交通违章事件,迅速地获取违章证据,提供行之有效的监测手段,为改善城市交
通拥堵现象起到了重要的作用,已成为道路交通管理队伍中必不可少的一员,以充分发挥它准确、公正的执法作用。 4、车联网(Internet of Vehicles):车联网是由车辆位置、速度和路线等信息构成的巨大交互网络。通过、、、摄像头等装置,车辆可以完成自身环境和状态信息的采集;通过技术,所有的车辆可以将自身的各种信息传输汇聚到中央处理器;通过技术,这些大量车辆的信息可以被分析和处理,从而计算出不同车辆的最佳路线、及时汇报路况和安排信号灯周期 5、自适应巡航ACC(Adaptivecruise control):自适应巡航控制系统是一种智能化的自动控制系统,它是在早已存在的巡航控制技术的基础上发展而来的。在车辆行驶过程中,安装在车辆前部的车距传感器(雷达)持续扫描车辆前方道路,同时轮速传感器采集车速信号。当与前车之间的距离过小时,ACC控制单元可以通过与制动防抱死系统、发动机控制系统协调动作,使车轮适当制动,并使发动机的输出功率下降,以使车辆与前方车辆始终保持安全距离。自适应巡航控制系统在控制车辆制动时,通常会将制动减速度限制在不影响舒适的程度,当需要更大的减速度时,ACC控制单元会发出声光信号通知驾驶者主动采取制动操作。当与前车之间的距离增加到安全距离时,ACC控制单元控制车辆按照设定的车速行驶。 6、车道偏移预警系统(Lanedeparture warning system):车道偏离预警系统是一种通过报警的方式辅助驾驶员减少汽车因车道偏离而发生交通事故的系统。车道偏离预警系统由图像处理芯片、控制器、传感器等组成。
充电桩建设实施计划方案
康巴什区充电桩(站)项 目 实 施 方 案
鄂尔多斯市尚普新能源有限公司 二○一七年七月 目录 一、项目背景 (2) 1.为什么要发展电动汽车 (2) 2.国家对电动汽车发展的政策支持 (2) 3.充电桩(站)的发展状况与趋势 (3) 二、公司基本概况 (5) 1.公司简介 (5) 2.公司的核心价值观、核心理念 (5) 3.公司的发展动力 (6)
4.公司的经营的口号 (6) 5.公司的经营范围 (6) 三、智能充电桩(站)建设实施方案 (7) 1.充电桩(站)充电用户对象分析 (7) 2.充电桩(站)的规划与敷设 (8) 3.充电桩(站)的分类及简介 (16) 4.充电桩(站)技术方案 (20) 5.充电桩(站)综合运营管理 (21) 6.新能源汽车与传统汽车的对比 (23) 四、智能电动自行车的充电建设方案 (24) 1.为什么发展电动自行车充电服务 (23) 2.电动自行车充电桩的特点 (25) 3.电动自行车充电桩支付方式及案例展示 (26) 五、充电桩(站)建设存在的问题 (30) 1.建设用地的开发成本过高 (30) 2.基础设施配套能力急需完善 (31) 3.充电设施投资成本高 (31) 4.充电设施运营效率低 (32) 5.充电设施盈利能力弱 (32)
六、康巴什区充电站的建设选址 (30) 1. 市政府建站效果 (34) 2.党校建站效果 (35) 一、项目背景 1.为什么要发展电动汽车 据英国石油公司发布的《BP世界能源统计2009》,全球原油剩余探明储量按照2008年的年开采速度计算,还可以开采42年。这意味着,到本世纪中叶,以电动汽车为代表的新能源汽车将毫无悬念地成为全球汽车工业的主导产品。 目前我国的石油对外依存度已经超过50%,电动汽车的发展将转变传统汽车的用能形式,可以大幅度地减少石油消耗,因此我国加快电动汽车的发展将成为改变能源消费结构、保障能源安全、振兴民族汽车工业的战略举措。 2.国家对电动汽车发展的政策支持 新政将行,推动力度 2014年7月13日,国家发 改委等五部委联合公布了《政府 机关及公共机构购买新能源汽
汽车发动机燃油供给系统教案
燃油供给系统 任务一汽油发动机燃料供给系统 学习目标 1.了解汽油机燃油系统的发展 2.掌握电控发动机燃油供给系统组成原理 3.掌握汽油机燃油供给系统组成部件作用 1.汽油机燃油系统的发展 上个世纪60年代,汽车用燃油输送系统绝大多数仍采用构造简化的化油器。随着汽车工业的发展,汽车尾气排放带来的空气污染日益严重,西方各国都制定了汽车排放法规法案。同时受能源危机的冲击以及电子技术、计算机等飞速发展,促进了电子控制汽油机喷射发动机的诞生。1953年美国奔第克斯(Bendix)首先开发了电子喷射器,1957年正式问世。 传统的化油器存在诸如发生气阻、结冰、节气门响应不灵敏等现象,在多缸发动机中供油不匀,引起工作不稳、不利于大功率设计。为了弥补这些缺陷,早在上世纪30年代,汽油喷射系统就已在开始航空发动机的研究中被作为研究对象,经过10多年的深入研发,在1945年开始应用于军用战斗机上。它充分的消除了浮子式化油器不能完全适用军用战斗机作战工况的缺点,汽油喷射技术应运而生。 尽管汽油喷射技术有诸多优势,但由于其生产受当时社会生产力、生产工艺、技术的制约,其制造成本非常高,因此汽车用汽油喷射装置最初只能应用在数量很少的赛车上,它能满足赛车所要求的大发动机输出功率和灵敏的油门响应性能。到50年代末期,大多数赛车都已经采用了汽油喷射作为燃油输送系统。 汽油喷射应用于民用批量生产的轿车发动机上,实在1950-1953年高利阿特与哥特勃罗特两公司首先在2缸2冲程发动机上安装了汽油喷射(缸内喷射)装置。1957年奔驰公司又在4冲程发动机上才用了它。 由于各发动机制造商强调发动机输出功率的提高,为了确保全负荷时大扭矩输出特性,空燃比控制必然偏小,以提高喷油量,因此,对空燃比的控制精度也比较低。但是随着电子控制技术的发展、应用,电子燃油控制的各种有点渐渐显现出来,包括各种精细的补偿功能和良好的空燃比控制性、灵敏的节气门响应性、高功率的从输出。 另外,在电子技术方面,晶体管早已发明,但是由于成本高,性能不稳定,还不能很好地应用于汽车上。故奔第克斯在开发阶段应用真空管开发了计算机。在1957年发表时,正式晶体管开始实用化时代,因此,她开发的电子控制汽油喷射装置只在美国三大汽车公司之一的克莱斯勒汽车上装用。 2.电控汽油机燃油喷射系统的优缺点 汽油喷射系统的实质就是一种新型的汽油供油系统。化油器利用空气流动时在节气门上方的喉管处产生负压,将浮子室的汽油连续吸出,经过雾化后输送给发动机。汽油喷射系统则是通过采用大量的传感器感受各种工况,根据直接或间接检测的进气信号,经过计算机判断和分析,计算出燃烧时所需的汽油量,然后将加有一定压力的汽油经过喷油器喷出,以供发动机使用。 电控发动机系统取消了化油器供油系中的喉管,喷油位置在节气门下方,直接在进气门
新能源汽车充电桩使用管理规定
新能源汽车充电桩使用管理规定 一、管理要求 1、车辆驶入充电位置,当车辆停稳,切断电动汽车动力电源和辅助电源,拉紧手刹,人员离车后,方可进行充电作业。 2、充电前,驾驶员应检查充电接口是否正常完好,并对车辆进行充电前检查,对充电设备与电动汽车连接和充电参数的设置进行确认。 3、充电启动后,确认充电正常,并定期巡视充电状态。发生安全事故,应快速按下红色急停按钮,切断电源。 4、充电过程中,车辆严禁启动或移动,严禁带电插拔充电插头。充电结束后、行车前,驾驶员应确认充电终止以及充电设备与电动汽车物理分离。 5、严禁使用金属物体触碰充电枪接口、纯电动汽车充电口。 6、驾驶员应基本了解电动汽车的构造和充电设备的工作原理,了解动力蓄电池应用的基础知识,掌握充电操作规程、充电设备检测、故障判断和处理、安全知识和应急处理方法。 7、驾驶员应按照充电桩生产厂家的顾客手册进行定期保养与例行检查,保持其安全、清洁、完好,并做好相关检查保养记录。 8、充电场所每日应做好日常检查,当班管理人员应对作业现场进行监督,发现违章行为和不安全因素,有权制止并向上级反映情况。充电作业人员应定期或根据工作需要随时进行巡视。 二、充电桩使用和管理 1、充电场所管理员必须定期检查充电桩及其他相关设备,消防器材、设施设备保持清洁干燥,并做相关检查记录。定时对充电场地、充电设备设施、消防器材保洁,确保设备处于良好状态。 2、充电过程中,驾驶员应按照操作流程操作,如厂家有其他充电要求,则按照厂家要求进行操作。同时须按要求对充电桩仪表、数据、充电模块、线路、开关等设施进行检查,并按要求填写巡检记录。 3、充电过程中如发生故障,充电场所管理员应立即按下充电机上的急停按键,以防故障进一步扩大,并上报技术科,由专业人员进行设备维修。 4、如遇系统起火时,首先动用紧急停机装置切断电源,然后使用干粉灭火器或者二氧化碳灭火器灭火,严禁使用泡沫灭火器和水灭火。 5、充电结束后,应按规定拔除充电枪,将线缆理好放在线架上,锁好充电口及车门,并记录相关数据。 6、严禁私自拆卸、改装充电桩设备及附加设施,对因此造成的损坏由当事人承担相应的经济责任,并对管理责任人进行问责。 三、充电场所管理 1、充电场所应设置安全管理组织机构,并配备专职或兼职的安全员,要建立安全管理制度和应急预案,建设安全应急保障体系。并负责制、修订充电场所管理责任书,负责指导、监督、评估充电场所安全管理工作。 2、充电场所管理人员是充电场所安全管理第一责任人,对充电场所安全进行管理。应建立运行值班制度,包括日常巡检、运行交接班、值班日志等。要落实、贯彻安全工作的相关规定及要求,组织开展充电场所安全实施工作,落实各级人员的应急职责。 3、露天设置的充电桩应有安全防护措施,保证雷雨等特殊天气的设备安全。 4、安全部门应定期组织充电桩厂家对站内充电桩进行巡检。 四、纯电动汽车充电管理
汽车辅助驾驶技术统计
汽车辅助驾驶统计 驾驶员辅助系统可以涵盖的功能有很多,包括:车道辅助、行车辅助、停车与操作辅助、避让辅助、转向与穿行辅助、照明与视野辅助等 博世驾驶员辅助系统涵盖了市场的需求与趋势,在必配功能方面包括自动紧急制动、车辆偏离警告等,标准功能包括自适应巡航、智能大灯控制等,除此之外还提供一些差异化功能如交通拥堵辅助、狭窄道路辅助等。 大陆集团的高级驾驶员辅助系统基于雷达、摄像机和红外传感器可以实现以下功能:紧急制动辅助;自适应巡航控制;车道偏离警告;智能前大灯控制;交通标志辅助;盲点探测和360度环绕检测(全景图)。 欧洲新车评价规程(EuroNCAP)规定,自2014年起,新车型必须装配相关驾驶员辅助系统才能获得五星安全评定。被列入配备选项的系统包括自动紧急制动、智能速度辅助、车道偏离警告或车道保持支持。
第一章浅析博世驾驶员辅助系统 ACC自适应巡航控制系统 ACC自适应巡航系统可以在道路中自动控制车速并保持与前车的距离。ACC使用雷达传感器发射电波并接收前方物体反射回的电波,根据反射回来的信号,ACC通过计算与相对距离、相对方位和相对速度来探测前方车辆,以作出加速或制动的判断。ACC可在车速约30km/h以上被激活,而停走型ACC可在静止时即可启用。 在ACC系统中,雷达传感器是最核心的部件。博世目前有两种雷达,一种为中距离雷达(MRR),可以探测160米的距离,可支持ACC最高巡航速度为150km/h,目前第七代高尔夫顶配车型上所使用的ACC系统就搭配了这款雷达,
性价比较高;博世长距离雷达(LRR)可以探测250米的距离,可支持ACC最高巡航速度为200km/h,如果该ACC系统搭配了多功能摄像头,最高巡航速度可达250km/h。奥迪A6L的停走型ACC在传统雾灯的位置装配了两部LRR,增加了探测的范围和距离。 ACC系统使用雷达传感器和多功能摄像机作为信息采集和输入端,可以在驾驶员不操作油门和刹车的情况下自动保持车距巡航,当前方车辆出现减速时随之刹停,而前方车辆离开时可自动加速至理想速度,在一定程度上接近了自动驾驶技术。不过,ACC并不能对车辆方向进行调整。 车道辅助系统/紧急制动系统 博世LDW车道偏离警告系统和LKS车道保持系统使用了一台多功能摄像头(MPC)进行车道线的识别,当系统识别到车道线时,自动进入工作状态。如果车辆在行驶中偏离了车道,且没有打转向灯,首先LDW会输出警告信号,而选择什么样的警告方式(如声音、仪表视觉符号以及方向盘振动等)由整车厂进行设定。如驾驶员没有回应,LKS系统将通过EPS电子转向系统在方向盘上施加大约3牛·米的力矩,以帮助车辆回到正确的车道上来。在这个过程中,如果驾驶员打方向灯或者大角度转动方向盘,则系统默认车辆由驾驶员接管而停止干预。
ADAS智能驾驶辅助系统
ADAS智能驾驶辅助系统 一、ADAS技术发展现状: 未来科技进步趋势将从“互联网”向“物联网”发展,智能驾驶是“万物互联”的最好载体,“无人驾驶”是汽车智能的终极发展方向。智能驾驶将进入高速发展期,预计在2020-2025年智能汽车将进入量产阶段,结合移动互联网、大数据、云计算的智能驾驶服务预计会在十年后全面推广。ADAS 是智能驾驶汽车的关键落地点,模块化分类主要有以下几点:车道偏离预警LDW,车道保持辅助LKA,紧急自动刹车AEB,智能远光灯IHC,自动泊车AP 等等。目前ADAS在国内外都属于研究阶段,只有一些高端车有了部分的技术储备,例如:丰田的公路自动驾驶辅助AHAC,特斯拉的自动巡航Autopilot,通用的Super Cruise。 二、ADAS技术市场格局分析: 智能驾驶技术未来的空间格局呈现金字塔结构,主要分为三层: 传统车企掌握着汽车生产资质和整车控制集成的核心竞争,科技型企业或者研究所凭借在人工智能、人机交互方面的优势抢占一部分市场份额。 ADAS供应商利用掌握的感知识别算法等为车企和科技型公司提供ADAS 系统解决方案; 底层零部件供应商:雷达,摄像头,芯片,电子刹车等等。 分析可知:底层零部件都掌握在供应商的手上,比较分散,其核心价值在于市场份额占据比例;塔尖的传统车企与科技公司,一般都会以合作的方式,核心产品大多为无人驾驶汽车这种涉及汽车生产资质与人工智能高端、核心算法的结合领域;中间层的ADAS研究是衔接二者的一个关键落地点,底层零部件是ADAS实现的载体,无人驾驶汽车是ADAS的高度集成。 ADAS技术领域的研究不仅仅可以作为塔尖与塔底的结合点,还可以通过ADAS技术的逐步深入研究与系统化集成,逐渐成为屹立于塔尖的科技型企业,从而实现整个技术点在质上的飞越与创新。 三、ADAS技术介绍: 1.整体框图:
新能源汽车充电桩建设
中国电动汽车充电桩已达2.8万个标准化仍未完成[摘要] 目前国内已经建成了723座充电站,28000个充电桩。但是产业链条上的各个利益方之间存有矛盾且并不妥协,也缺乏合适实施的统一规则。 充电桩、充电站的建设程度,直接影响着电动汽车的大规模商业化推广,而仅仅增加充电设施的布局并不够,还需建设高效完善的充电服务网络。 事实上,目前有实力的生产厂商都希望自己研究的标准能够代表行业的标准,各家都认为自己掌握了较为核心的技术,并且不会将这些轻易对外开放,都希望公司能够将上游的电动汽车生产销售到下游充电技术和充电桩建设融于一身,打造一个全产业链的公司。 723座充电站,28000个充电桩。这是工信部关于目前我国国内充电站和充电桩最新的统计。而这两个数字还在快速更新着。 2014年,国际知名电动汽车品牌特斯拉进入中国市场,炫酷的外观和搭载的动力系统给中国消费者和众多汽车企业带去了视觉和思想上的冲击。 随后,新能源汽车企业在沉淀了相当的试验产品后,开始如雨后春笋般纷纷冒出,众多生产商推出各自的电动汽车产品来争夺这个细分的市场。 充电桩、充电站的建设程度,直接影响着电动汽车的大规模商业化推广,而仅仅增加充电设施的布局并不够,还需建设高效完善的充电服务网络。 1月27日,在一次例行的新闻发布会上,工业和信息化部运行监测协调局局长郑立新对外透露:“2014年新能源汽车得到了快速发展,全年共生产了83900辆新能源汽车。”
产业的蓝海效应自然引起各方的浓厚兴趣。但是记者了解发现,数据快速增长的背后,充电桩行业正进行着“野蛮的生长”。产业链条上的各个利益方之间存有矛盾且并不妥协,也缺乏合适实施的统一规则。 电桩产业:在质疑中快速增长 充电桩和充电站这一重要配套产业的完善将促进电动汽车的发展。和电动汽车产业一样,充电桩在全国的建设也在不断创造着新的记录。 工信部1月27日公布的最新数据显示,目前国内已经建成了723座充电站,28000个充电桩。其中,国家电网公司建成充换电站618座,充电桩2.4万个。根据十三五规划,预计到2020年,集中式充换电站将增长到1.2万座,分散式充电桩数量更将增长100倍达到450万个。 正是基于“充电设施建设网络化、规模化”的发展理念,1月中旬,国内首个高速公路跨城际快充网络——京沪高速公路快充网络全线贯通,成为了充电桩产业发展过程中的里程碑事件。 国家电网方面提供的数据显示,此条高速沿线建成50座快充站,平均单向每50公里一座快充站。每座快充站规划建设4台120千瓦直流充电机、8个充电桩,可同时为8辆电动汽车充电,30分钟内充满(80%电量),先期建设2台充电机、4个充电桩,支持所有符合中国标准的电动汽车充电。 不过,记者从一些特斯拉的车主处证实,京沪高速快速充电网络贯通后,国内一些使用了国电技术的国产电动汽车车友,自发组织了一次体验京沪充电网络的活动,部分路段的高速充电站均不能为电动车充电,并且以失败而告终。因而引发车主质疑“贯通”之说。
电动汽车充电桩建设项目
AA县电动汽车充电桩建设项目可行性研究报告 AA县BB公交有限责任公司 二〇一五年十一月
目录 第一章总论 (1) 1.1项目概况 (1) 1.2编制依据 (5) 1.3结论 (7) 第二章项目建设背景及必要性 (8) 2.1项目建设背景 (8) 2.2项目建设的必要性 (13) 第三章市场分析 (16) 3.1市场现状分析 (16) 3.2市场前景 (18) 第四章项目区概况及场址选择 (20) 4.1项目区概况 (20) 4.2场址选择 (21) 第五章技术、设备方案、工程方案 (23) 5.1技术方案 (23) 5.2工程方案 (29) 5.3设备方案 (30) 第六章总图布置与公用工程 (32) 6.1总图布置 (32) 6.2公用工程 (33) 第七章节能节水措施 (37) 7.1用能标准和节能规范 (37) 7.2能源消耗状况 (38) 7.3节能措施 (38) 7.3节水措施 (39) 7.4节能管理 (40) 第八章环境保护 (41) 8.1环境保护依据 (41) 8.2主要污染物及环保措施 (41) 8.3环境保护结论 (45) 第九章劳动安全卫生与消防 (46) 9.1劳动安全卫生 (46) 9.2消防设施 (50)
第十章项目管理与实施 (53) 10.1项目管理 (53) 10.2劳动定员 (53) 10.3项目实施安排 (54) 10.4项目招标 (54) 第十一章投资估算与资金筹措 (56) 11.1投资估算 (56) 11.2资金筹措 (60) 第十二章效益分析 (61) 12.1经济效益 (61) 12.2社会效益 (63) 第十三章结论与建议 (65) 13.1结论 (65) 13.2建议 (66)
燃料电池汽车的动力传动系统设计
燃料电池汽车的动力传动系统设计 1引言 燃料电池汽车是电动汽车的一种。 燃料电池发出的电,经逆变器、控制器等装置,给电动 机供电,再经传动系统、驱动桥等带动车轮转动 ,就可使车辆在路上行驶,燃料电池的能量转 换效率比内燃机要高 2-3倍。燃料电池的化学反应过程不会产生有害产物 ,因此燃料电池车 辆是无污染汽车。随着对汽车燃油经济性和环保的要求 ,汽车动力系统将从现在以汽油等化 石燃料为主慢慢过渡到混合动力 ,最终将完全由清洁的燃料电池车替代。 近几年来,燃料电池系统和燃料电池汽车技术已经取得了重大的进展。世界著名汽车制 造厂,如丰田、本田、通用、戴姆勒-克莱斯勒、日产和福特汽车公司已经开发了几代燃料电 池汽车,并宣布了各种将燃料电池汽车投向市场的战略目标。 目前,燃料电池轿车的样车正在 进行试验,以燃料电池为动力的运输大客车在北美的几个城市中正在进行示范项目。其中本 田的FCX Clarity 最高时速达到了 160 km/h[8];丰田燃料电池汽车 FCHV-adv 已经累计运行 了 360,000 km 的路试,能够在零下37度启动,一次加氢能够从大阪行驶到东京 (560公 里)。 在我国科技部的支持下,燃料电池汽车技术得到了迅速发展。 2007年,我国第四代燃料电池 轿车研制成功,该车最高时速达150 km/h,最大续驶里程319 km 。2008年,20燃料电池示范 汽车又 在北京奥运进行了示范运行。 2010年,包括上汽、奇瑞等国内汽车企业共有 196辆燃 料电池汽车在上海世博园区进行示范运行。 燃油绘济性 排放环保 l ;uel economic exhaust eih ironmen(al protection Internal combustion engine Shori peicxl Mid peitxl Long pei 汽车智能辅助驾驶系统 目录 1 需求分析……………………………………… (1) 2 智能车和智能交通系统简介 (1) 3 CCD摄像头的图像采集原理 (2) 4 图像的预处理……………………………………… (3) 5道路区域检测……………………………………… (4) 6目标检测和车距测量……………………………………… (5) 7系统的硬件构成和工作原理……………………………………… 6 8系统软件流程图……………………………………… (7) 9结论与展望……………………………………… (8) 10参考文献……………………………………… (9) 需求分析 汽车作为一种快速、灵活而经济的交通工具,普遍受到人们的关注。20世纪后半叶以来,汽车工业得到了迅速发展。国家积极推进汽车工业和消费,汽车进入寻常百姓家。但是汽车给我们带来方便的同时也带来了不少的问题,其中最主要的就是交通事故频繁发生,由此导致的人员伤亡和财产损失数目嘛人。据全球各交通和警察部门的统计:2003年全世界交通事故死亡人数为50万人,其中,中国交通事故死亡人数为l0.4万人,占世界交通事故死亡人数的20%还多,而美国、俄罗斯的死亡人数则分别为4万人和2.6万人;拿两个规模相当的城市比较,北京的交通事故致死率为14%,东京则为0.7%。在诸多交通事故中,由于驾驶员反应不及 1 造成的交通事故占80%以上,汽车追尾事故占30%一40%,而追尾事故造成的损失和伤亡又占总损失的60%以上。据奔驰汽车公司的一项研究表明:驾驶员只要在有碰撞危险的0.5秒前得到预警,就可以避免至少60%的追尾撞车的事故,30%的迎面撞车事故和50%的路面相关事故;若在1秒前“预警”,则可避免90%的事故发生。中国正在成为全球最大的新兴市场,汽车保有量已突破2600万辆,年销售汽车将突破600万辆,未来5年将成为仅次于美国的全球第二大汽车销售国。而纵观世界汽车的数量则更是多得惊人,光是美国国内的汽车保有量就多达2亿多辆,并且世界每年还有成亿的新车涌向市场。如此巨大的汽车数量和汽车市场,加上极端残酷的车祸事故和悲惨后果,发展汽车安全技术刻不容缓。汽车安全技术主 2汽车智能辅助驾驶系统
