立方形氧化钴的合成及其形状的控制(英文)

Shape-controlled synthesis of nanocubic Co3O4 by
hydrothermal oxidation method
YANG You-ping(杨幼平), HUANG Ke-long(黄可龙), LIU Ren-sheng(刘人生),
WANG Li-ping(王丽平), ZENG Wen-wen(曾雯雯), ZHANG Ping-min(张平民) School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 30 December 2006; accepted 9 April 2007
Abstract: The nanocubic Co3O4 was synthesized by hydrothermal oxidation method. The effects of cobalt salt, precipitating agent, surfactant, solvent, pH value of the suspension and the amount of oxidant H2O2 on the morphology and structure of Co3O4 were investigated. The Co3O4 powders were characterized by transmission electron microscope and X-ray diffraction. The results show that the morphology of Co3O4 is closely dependant on the anion in cobalt salts, but it is not so sensitive to the precipitating agents and solvents. The amount of H2O2 is the key factor to obtain Co3O4 with spinel crystal structure. The optimum synthetic conditions of uniform shape-controlled Co3O4 nanocubes are as follows: Co(CH3COO)2·4H2O as cobalt salt, KOH as precipitating agent, polyethylene glycol with relative molecular mass of about 20 000 as surfactant, water?n-butanol as solvent system, pH value of 8?9, the molar ratio of H2O2 to Co2+ above 2.5?1.0, hydrothermal temperature of 160 ℃ and hydrothermal holding time of 10 h. The tap density and apparent density of nanocubic Co3O4 obtained with the average particle size of 20 nm are 1.01 g/cm3 and 0.70 g/cm3, respectively.
Key words: Co3O4; nanocubes; shape-controlled;hydrothermal oxidation
1 Introduction
The tricobalt tetraoxide Co3O4 belongs to the normal spinel crystal structure based on a cubic close packing array of oxide atoms, in which Co2+ ions occupy the tetrahedral 8a sites and Co3+ ions occupy the octahedral 16d sites. In recent years, Co3O4 has attracted attention due to its wide applications in catalysts[1], magnetic semiconductors[2], electrode material[3?5], gas sensors[5] and pressure sensitive ceramics[6]. Various methods, such as the thermal decomposition of solid phase[7?8], sol-gel method[9], hydrothermal method[10?11], solvothermal decomposition[12], chemical vapor deposition[13], liquid-control- precipitation method[14] and spray pyrolysis[15], were attempted to synthesize nanosized spinel Co3O4. It is well-known that the behaviors of nanophase materials strongly depend on the shape and size of the particles[5]. And hydrothermal oxidation method is an efficient technique for preparing fine uniform particles of metal oxides[16].
ZHANG et al[10] studied the effects of hydro- thermal synthetic conditions, such as the starting concentration of Co(NO3)2 solution, pH value, hydrothermal temperature, holding time and the stocking mode, on the shape and size of Co3O4 cubes in Co(NO3)2-NH3·H2O system. The Co3O4 and β-Co(OH)2 mixtures were obtained when the temperature was below 180 ℃ and hydrothermal holding time was 1?36 h, and the cubic Co3O4 could be obtained by calcining the mixtures in air. JIANG et al[11] reported that Co(OH)2 gel, which was prepared using CoSO4·7H2O and NH3·H2O as starting materials, could be oxidized to nanocrystalline Co3O4 by hydrogen peroxide in a hydrothermal system at 180 ℃ for 24 h. Although Co(OH)2 gel was filtered using vacuum filtration and washed by distilled water for several times until no and remained, the morphology of nanocrystalline Co3O4 was irregular. In order to synthesize uniform shape-controlled Co3O4 nanocubes, the effects of anion in cobalt salt, precipitating agent, surfactant, solvent, pH value of the suspension, and the amount of oxidant H2O2 on the morphology and structure ?
2
4
SO+4
NH
Foundation item: Project(50542004) supported by the National Natural Science Foundation of China; Project(ZE097) supported by Creative Program of Central South University, China
Corresponding author: HUANG Ke-long; Tel: +86-731-8879850; E-mail: klhuang@https://www.360docs.net/doc/a49295088.html,
YANG You-ping, et al/Trans. Nonferrous Met. Soc. China 17(2007) 1083
of Co3O4 were investigated in this study.
2 Experimental
15 mmoL cobalt salt was dissolved into distilled water containing certain surfactant and organic solvent, and then excessive amount of precipitating agent was added with electromagnetic stirring at 30 ℃ during the formation of Co(OH)2 precursor. The pH value of the suspension after precipitation reaction was monitored to 8?9. A certain amount of 30% (mass fraction) H2O2 was dropped into the suspension. Finally, all of them were transferred into a Teflon-lined stainless steel autoclave with the volume of 100 mL, and the autoclave was filled with distilled water up to 70% of the total capacity. The sealed autoclave was heated to 160 ℃ and maintained for 10 h, then cooled to room temperature in air naturally. The black powders were centrifuged and washed with distilled water and absolute ethanol for three times, respectively, and dried in an oven at 80 ℃ for 6 h.
The morphology and size of the obtained powders were determined by using a Japan JEOL JEM?1230 transmission electron microscopy(TEM). The X-ray diffraction(XRD) patterns of the powders were obtained with a Japan Rigaku D/max?2500 X-ray diffractometer using Cu Kα radiation in the 2θrange from 10? to 80?.
3 Results and discussion
3.1 Effect of cobalt salt and precipitating agent
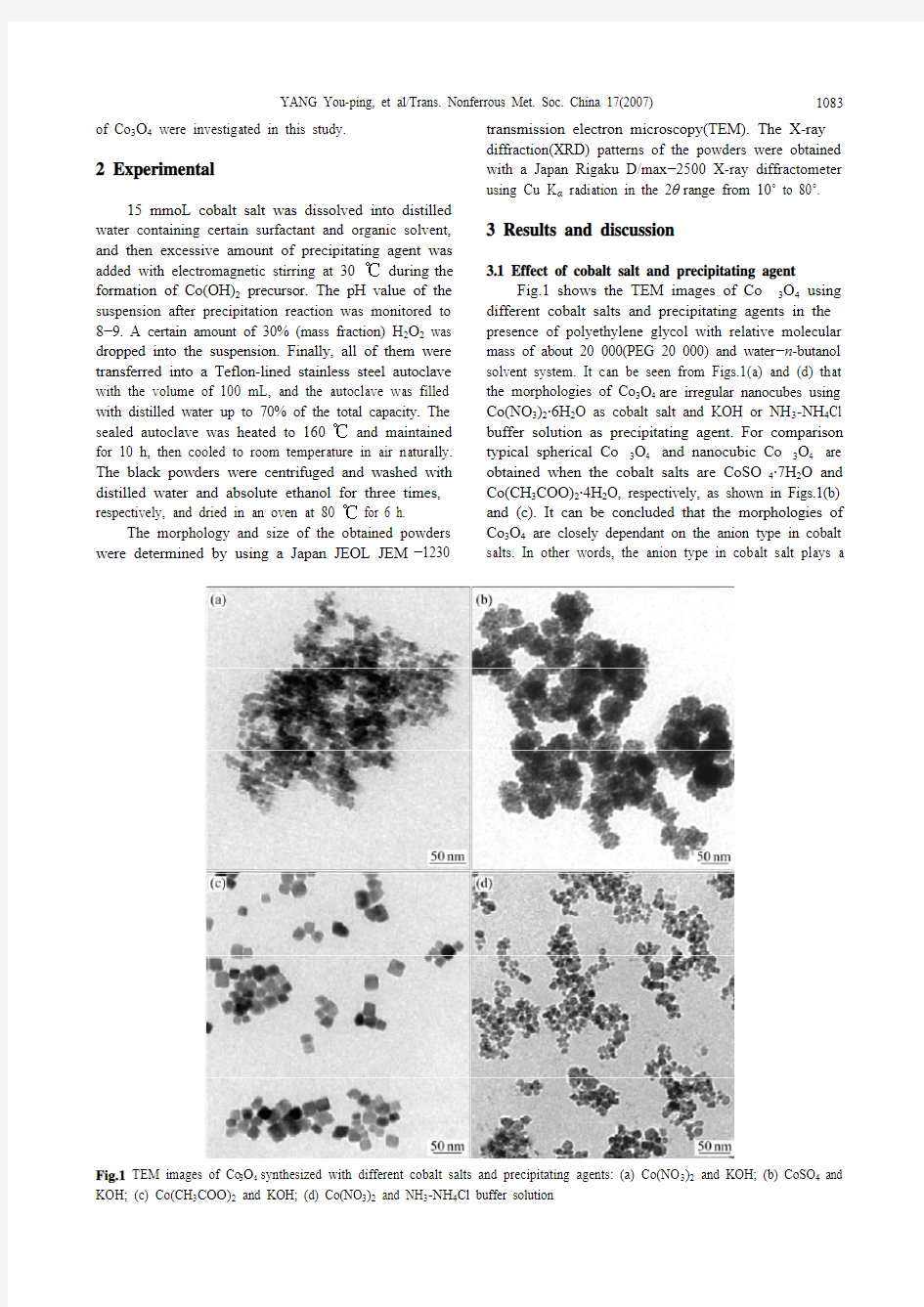
Fig.1 shows the TEM images of Co3O4 using different cobalt salts and precipitating agents in the presence of polyethylene glycol with relative molecular mass of about 20 000(PEG 20 000) and water?n-butanol solvent system. It can be seen from Figs.1(a) and (d) that the morphologies of Co3O4 are irregular nanocubes using Co(NO3)2·6H2O as cobalt salt and KOH or NH3-NH4Cl buffer solution as precipitating agent. For comparison typical spherical Co3O4 and nanocubic Co3O4 are obtained when the cobalt salts are CoSO4·7H2O and Co(CH3COO)2·4H2O, respectively, as shown in Figs.1(b) and (c). It can be concluded that the morphologies of Co3O4 are closely dependant on the anion type in cobalt salts. In other words, the anion type in cobalt salt plays a
Fig.1 TEM images of Co3O4 synthesized with different cobalt salts and precipitating agents: (a) Co(NO3)2 and KOH; (b) CoSO4 and KOH; (c) Co(CH3COO)2 and KOH; (d) Co(NO3)2 and NH3-NH4Cl buffer solution
YANG You-ping, et al/Trans. Nonferrous Met. Soc. China 17(2007) 1084
key role in the morphology of Co3O4, while the influence
of precipitating agent on the morphology of Co3O4 is very limited. Therefore, Co(CH3COO)2·4H2O and KOH are chosen as cobalt salt and precipitating agent to synthesize nanocubic Co3O4, respectively.
3.2 Effect of surfactant
Fig.2 shows the effects of surfactant on morphology
of Co3O4 in water?n-butanol solvent system. It can be seen that nanocubic Co3O4 synthesized in the presence of non-ionic surfactant PEG 20 000 is highly dispersed and shows excellent uniformity, while Co3O4 nanoparticles obtained from anionic surfactant sodium dodecyl benzene sulfonate(SDBS) are agglomerated in irregular shapes. This may be due to the interface retarding effect
of PEG 20 000. The relative molecular mass of PEG 20 000 is greater than that of SDBS. As a result, PEG 20 000 is chosen as the surfactant in the synthesis of nanocubic Co3O4.
3.3 Effect of pH value
Fig.2 TEM images of Co3O4 synthesized with different surfactants: (a) Polyethylene glycol 20 000; (b) Sodium dodecyl benzene sulfonate
Fig.3 shows the TEM image of Co3O4 synthesized in suspension of pH 11?12 after precipitation reaction. Compared Fig.1(c) with Fig.3, it is very obvious that nanocubic Co3O4 with the average particle size of 20 nm is formed when pH is 8?9, and when pH goes up to 11?12 irregular Co3O4 including some grains recombined in the products becomes serious. Because the condensation reaction of Co(OH)2 precursor can easily occur at higher pH value, agglomeration of the nanoparticles occurs.
Fig.3 TEM image of Co3O4 synthesized in suspension of pH 11?12
The condensation reaction of Co(OH)2 can be xpressed as
e
—Co—O—H+H—O—Co—→—Co—O—Co—+H2O
(1)
In order to synthesize nanocubic Co3O4, the pH value of suspension should be strictly controlled at 8?9.
3.4 Effect of solvent
The XRD patterns of Co3O4 synthesized in different solvent systems are shown in Fig.4. All peaks shown in Fig.4 can be indexed to a cubic spinel crystal structure Co3O4. No impurity peaks are observed, which indicates that the final product synthesized is Co3O4 with spinel crystal structure under hydrothermal oxidation condition. Based on Scherrer formula, the average particle sizes of Co3O4 in water, water?alcohol and water?n-butanol solvent systems are calculated to be 27 nm, 10 nm and 15 nm, respectively.
Fig.5 shows the TEM images of Co3O4 synthesized in water and water?alcohol solvent systems using Co(CH3COO)2·4H2O as cobalt salt. Compared Fig.1(c) with Fig.5, it can be seen that nanocubic Co3O4 particles are all obtained in these solvent systems. While Co3O4 synthesized in water?n-butanol solvent system shows a
YANG You-ping, et al/Trans. Nonferrous Met. Soc. China 17(2007) 1085
Fig.4 XRD patterns of Co3O4 synthesized in different solvent systems: (a) Water; (b) Water–alcohol; (c) Water?n-butanol
Fig.5 TEM images of Co3O4 synthesized in different solvent systems: (a) Water; (b) Water?alcohol
better monodisperse sign (Fig.1(c)), and the tap density and apparent density of uniform shape-controlled Co3O4 nanocubes are 1.01 g/cm3 and 0.70 g/cm3, respectively.3.5 Effect of amount of H2O2
In order to get Co3O4 with spinel crystal structure, the amount of oxidant H2O2 should be enough. The chemical reaction in the hydrothermal oxidation process an be expressed as
c
3Co(OH)2+H2O2→Co3O4+4H2O (2) So the molar ratio of H2O2 to Co(OH)2 is 1?3 in theory. However, H2O2 tends to decompose in the practical operation, therefore the amount of H2O2 is far more than the theoretical value.
Fig.6 shows the XRD patterns of the samples obtained with adding different amount of H2O2. When the molar ratio of H2O2 to Co2+ is 2.0?1.0, the impurity Co(OH)2 still exists. While the molar ratio of H2O2 to Co2+ is increased to 2.5?1.0, Co3O4 with cubic spinel crystal structure is obtained. So in order to obtain Co3O4 with spinel crystal structure, the molar ratio of H2O2 to Co2+ should be higher than 2.5?1.0.
Fig.6 XRD patterns of Co3O4 synthesized with different molar ratios of H2O2 to Co2+: (a) 2.0?1.0; (b)2.5?1.0
4 Conclusions
1) The uniform shape-controlled spinel Co3O4 nanocube is prepared by hydrothermal oxidation method. The optimum synthetic conditions of Co3O4 nanocubes are as follows: Co(CH3COO)2·4H2O as cobalt salt, KOH as precipitating agent, polyethylene glycol 20 000 as surfactant, pH value of 8?9,molar ratio of H2O2 to Co2+ above 2.5?1.0, hydrothermal temperature of 160 ℃ and hydrothermal holding time of 10 h.
2) The morphology of Co3O4 is closely dependant on the anion in cobalt salts. The nanocrystalline, spherical and uniform nanocubic Co3O4 particles are obtained using Co(NO3)2·6H2O, CoSO4·7H2O and Co(CH3COO)2·4H2O as cobalt salts, respectively.
3) The precipitating agent and solvent system have little influence on morphology of Co3O4. The Co3O4 nanocubes are all synthesized in water, water?alcohol
YANG You-ping, et al/Trans. Nonferrous Met. Soc. China 17(2007) 1086
and water?n-butanol solvent systems, and the average particle sizes of Co3O4 are calculated to be 27, 10 and 15 nm, respectively. The tap density and apparent density of uniform shape-controlled Co3O4 nanocubes synthesized in water?n-butanol solvent system are 1.01 g/cm3 and 0.70 g/cm3, respectively.
4) The amount of H2O2 is the key factor to obtain Co3O4 with spinel crystal structure. The molar ratio of H2O2 to Co2+ should be higher than 2.5?1.0. References
[1]WANG Chen-bin, TANG Chih-wei, GAU Shiue-Jiun, CHIEN
Shu-hua. Effect of the surface area of cobaltic oxide on carbon monoxide oxidation [J]. Catalysis Letters, 2005, 101(1/2): 59?63. [2]ICHIYANAGI Y, KIMISHIMA Y, YAMADA S. Magnetic study on
Co3O4 nanoparticles [J]. Journal of Magnetism and Magnetic Materials, 2004, 272: e1245?e1246.
[3]WANG G X, CHEN Y, KONSTANTINOV K, LINDSAY M, LIU H
K, DOU S X. Investigation of cobalt oxides as anode materials for
Li-ion batteries [J]. Journal of Power Sources, 2002, 109: 142?147. [4]YUAN Zheng-yong, HUANG Feng, FENG Chuan-qi, SUN Ju-tang,
ZHOU Yun-hong. Synthesis and electrochemical performance of nanosized Co3O4 [J]. Materials Chemistry and Physics, 2003, 79: 1?4.
[5]LI Wei-yang, XU Li-na, CHEN Jun. Co3O4 nanomaterials in
lithium-ion batteries and gas sensors [J]. Advanced Functional Materials, 2005, 15(5): 851?857.
[6]CAO Quan-xi, ZHOU Xiao-hua, CAI Shi-dong. The function and
effect of Co3O4 on suppress sensitive ceramics [J]. Piezoelectrics and
Acoustooptics, 1996, 18(4): 260?263. (in Chinese)
[7]ARDIZZONE S, SPINOLO G, TRASATTI S. The point of zero
charge of Co3O4 prepared by thermal decomposition of basic cobalt
carbonate [J]. Electrochimca Acta, 1995, 40(16): 2683?2686.
[8]LIAO Chun-fa, LIANG Yong, CHEN Hui-huang. Preparation and
characterization of Co3O4 by thermal decomposition from cobalt oxalate [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(12):
2131?2136. (in Chinese)
[9]CAO Jin-zhang, ZHAO Yan-chun, YANG Wu, TIAN Jian-niao,
GUAN Fei, MA Yong-jun. Sol-gel preparation and characterization
of Co3O4 nanocrystals [J]. Journal of University of Science and Technology Beijing, 2003, 10(1): 54?57.
[10]ZHANG Wei-min, SONG Xin-yu, LI Da-zhi, YU Hai-yun, SUN
Si-xiu. Effects of hydrothermal synthetic conditions on Co3O4 cubes
morphologies [J]. Chemical Journal of Chinese University, 2004, 25(5): 797?801. (in Chinese)
[11]JIANG Yang, WU Yue, XIE Bo, XIE Yi, QIAN Yi-tai. Moderate
temperature synthesis of nanocrystalline Co3O4 via gel hydrothermal
oxidation [J]. Materials Chemistry and Physics, 2002, 74: 234?237. [12]NETHRAVATHI C, SEN S, RAVISHANKAR N, RAJAMATHI M,
PIETZONKA C, HARBRECHT B. Ferrimagnetic nanogranular Co3O4 through solvothermal decomposition of colloidally dispersed
monolayers of α-cobalt hydroxide [J]. J Phys Chem B, 2005, 109(23): 11468?11472.
[13]BAHLAWANE N, FISCHER RIVERA E, KOHSE-HOINGHAUS K,
BRECHLING A, KLEINEBERG U. Characterization and tests of
planar Co3O4 model catalysts prepared by chemical vapor deposition
[J]. Applied Catalysis B: Environmental, 2004, 53: 245?255.
[14]LI Ya-dong, HE Yun-pu, LI Long-quan, QIAN Yi-tai. Fabrication of
Co3O4 ultrafines by a liquid-control-precipitation method [J].
Chemical Journal of Chinese University, 1999, 20(4): 519?522. (in
Chinese)
[15]SHINDE V R, MAHADIK S B, GUJAR T P, LOKHANDE C D.
Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis
[J]. Applied Surface Science, 2006, 252: 7487?7492.
[16]XU Ru-ren, PANG Wen-qin. Inorganic synthesis and preparative
chemistry [M]. Beijing: Higher Education Press, 2001. (in Chinese)
(Edited by LI Xiang-qun)
自动化专业英文自我介绍
自动化专业英文自我介绍 General Introduction* I am a third year master major in automation at Shanghai Jiao Tong University, P. R. China. Education background In 1995, I entered the Nanjing University of Science & Technology (NUST) -- widely considered one of the China’s best engineering schools. During the following undergraduate study, my academic records kept distinguished among the whole department. I was granted First Class Prize every semester, and my overall GPA(89.5/100) ranked No.1 among 113 students. In 1999, I got the privilege to enter the graduate program waived of the admission test. I selected the Shanghai Jiao Tong University to continue my study for its best reputation on Combinatorial Optimization and Network Scheduling where my research interest lies. At the period of my graduate study, my overall GPA(3.77/4.0) ranked top 5% in the department. In the second semester, I became teacher assistant that is given to talented and matured students only. This year, I won the Acer Scholarship as the one and only candidate in my department, which is the ultimate accolade for distinguished students endowed by my university. Presently, I am preparing my graduation thesis and trying for the honor of Excellent Graduation Thesis. Research experience and academic activity
很好听的100首外语歌
很好听的100首外 语歌草帽小子整理 1. Moves like jagger (来自maroon 5 和christina,很动感,很好听,百听不厌。不过我已经厌了,因为听了不止百遍啦!建议看这首歌在《美国之声》的首发现场版,个人感觉比MV好) 2 .Die young (整首歌都很动感,感觉比Tik Tok 更好。第一次听就觉得它能登上Billboard榜首,但当时恰逢美国一场伤亡较大的校园枪击案,这首《英年早逝》太应景了,点击率直降。也只能说Kesha运气不好吧!) 3. We are young (这首歌让我彻底爱上FUN.,MV拍的也不错,骑车的时候听这首歌非常好) 4. Halo (Beyonce 的歌中我最爱的一首,现场版非常好,吉克隽逸翻唱的也很棒) 5.Just give me a reason (最初听是在首发现场秀,没觉得特好听,后来它在billboard 表现太抢眼,再次听就爱上了。本来我就非常喜欢FUN.的主唱Nate Russ,觉得Pink有些模仿他唱歌风格) 6.Stay (初次听了一点就听不下去了,机缘巧合之下听了完整版,OMG!太好听了,我喜欢的风格。也是Rihanna 的歌中最喜欢的一首.很希望她能翻唱Patti Austin 的say you love me ,我很喜欢那歌词。因为我觉得rihanna 能胜过austin,听了stay之后) 7.oh Aaron! (《天之骄子》最喜欢的两首歌之一,另一首是《not too young not too old》。都是carter 兄弟两合作的,身为后街男孩主唱的nick友情帮忙。nick
案例分析采购与付款业务循环内部控制
[优质文档]案例分析—釆购与付款业务循环内部控制1 采购与付款循环业务环节案例分析 B公司主要经营中小型机电类产品的生产和销售,U前主要采用手工会计系统。通过对B公司内部控制的了解,记录了所了解的和购货与付款循环的内部控制程序,部分内容摘录如下: (1)对需要购买的已经列入材料清单基础上的山仓库负责填写请购单,对未列入存货清单基础上的山相关需求部门填写请购单。每张请购单须山对该类采购支出预算负责的主管人员签字批准。 (2)釆购部收到经批准的请购单后,山其职员E进行询价并确定供应商,再由其职员F负责编制和发出预先连续编号的订购单。订购单一式四联,经被授权的采购人员签字后,分别送交供应商、负责验收的部门、提交请购单的部门和负责采购业务结算的应付凭单部门。(3)釆购人员F根据请购单向公司的长期供应商C公司发出订购单,釆购人员F长年以来一直负责向C公司采购材料。 (4)根据仓库部门记录,C公司虽然经常出现交货不及时、数量不符等问题,但山于从C公司采购的材料的价格相对较低,因此财务部门指定C公司为B 公司材料的主要供应商。(5)验收部门根据订购单上的要求对所采购的材料进行验收,完成验收后,将原材料交山仓库人员存入库房,并编制预先未连续编号的验收单交仓库人员签字确认。验收单一式三联,其中两联分送应付凭单部门和仓库,一联留存验收部门。 (6)对于验收部门发现的存在质量问题的材料,B公司要求采购部 门与C公司进行谈判并确定适当的折让金额,并授权财务经理审批折让金
额,折让金额一经确定,财务部门即应编制贷项凭单,调整应收账款。 (7)应付凭单部门核对供应商发票、验收单和订购单,并编制预先连续编号的付款凭单。在付款凭单经被授权人员批准后,应付凭单部门将付款凭单连同供应商发票及时送交会计部门,并将未付款凭单副联保存在未付款凭单档案中。会计部门收到附供应商发票的付款凭单后即应及时编制有关的记账凭证,并登记原材料和应付账款账簿。(8)应付凭单部门负责确定尚未付款凭单在到期日付款,并将留存的未付款凭单及其附件根据授权审批权限送交审批人审批。审批人审批后,将未付款凭单连同附件交复核人复核,然后交财务出纳人员Jo出纳人员J据此办理支付手续,登记现金和银行存款日记账,并在每月末编制银行存款余额调节表,交会计主管审核。 (9)每月月末,财务经理授权负责付款的出纳人员J负责定期核对供应商的订单,针对发现的任何差异,追查本公司的会计记账是否有误,并与供应商及时联系,调整差异。 试逐一判断上述每一业务环节是否有内控缺陷? 答案: (1)不存在内控缺陷,因为对于仓库负责对列入清单的货物填写请购单,同时如果没有列入存货清单的话则可以山其他部门根据需要填写。但是每张请购单要山经过该类支付负预算责任的主管人员签字批准。 (2)存在内部控制缺陷,因为对于询价和确定供应商是属于不相容的 两个岗位,而该公司却由一人担任是不正确的。应当建议其由另外一个职员负责确定供应商。 (3)存在内部控制缺陷,因为企业应根据具体惜况对办理采购业务的 人员进行定期轮岗,防范采购人员利用职权和工作便利收受商业贿赂,损害企
机械式温度控制器原理
机械式温度控制器原理 机械式温度控制器实际上是一种压力式(气压式)温度控制器,其控温原理如图5-6所示。图5-6 机械式温度控制器的控温原理 从结构上看,机械式温度控制器主要由感温器和触点式微型开关组成。其中,感温器叫做温压转换部件,它是一个封闭的囊体,主要由感温头、感温管和感温腔三部分组成。根据感温腔的形式不同,感温器又分为波管式和膜盒式两种感温头位于蒸发器的表面或电冰箱箱体内,用以感应电冰箱箱内的温度。感温管内充有感温剂,温度控制器旋钮用以设定电冰箱的制冷温度。 当蒸发器表面的温度上升并超过温度控制器旋钮设定 的温度时,感温管内感温剂的压力增大,感温腔中的隔膜在压力的作用下压迫传动支板,使触点接通,电路闭合,压缩机开始运转,电冰箱开始制冷。当蒸发器表面的温度逐渐下降至设定值时,感温管内感温剂的压力下降,弹簧的收缩力大于感温腔隔膜对传动支板产生的推力,传动支板即在弹簧的收缩作用下微微向上抬起,使得触点断开,压缩机便随之停止运转。 电冰箱制冷温度的调节是通过调节温度控制器旋钮实 现的。当调整温度控制器旋钮时,温度控制器旋钮便带动调
温凸轮转动,从而使温度控制板控制弹簧的张力。 图5-7为温度控制器的调温凸轮与温度控制板的关系示意图。图5-7 调温凸轮与温度控制板的关系示意图 调整温度控制器旋钮时,旋钮的转动实际上就带动调温凸轮转动,便会造成温度控制板的前移或后移,从而控制弹簧拉力的增大或缩小。若弹簧拉力较大,就需要待蒸发器温度较高时使感温剂压力增大,产生较大的推动力使得传动支板前移,推动触点闭合,压缩机才会启动工作。这就是调高电冰箱温度的方法。反之,若弹簧拉力较小,当蒸发器温度稍微升高时,感温剂所产生的压力就足以推动传动支板,使触点闭合,启动压缩机工作,这样就将电冰箱的制冷温度调低了。 图5-7中的温度调节螺钉是用来调整温度范围的,将该螺钉顺时针转动(右旋),相当于加大了弹簧的拉力,使得 温控点升高。如果电冰箱出现不停机的故障,可将该调节螺钉顺时针旋转半周或一周。反之,若逆时针转动该温度调节螺钉(左旋),则相当于减小弹簧的拉力,使得温控点降低。当电冰箱出现不能规律性启动的故障时,可将该调节螺钉逆时针旋转半周或一周。 值得注意的是,电冰箱温度的调节是否合理直接关系到其使用寿命。电冰箱的工作时间和耗电量受周围环境的影响很大。通常在夏季时,周围的环境温度较高,这时最好不要将电冰
采购业务内控制度试行
采购业务内控制度(试行)1 采购业务的内部控制制度(试行) 一、采购业务的授权 公司的采购业务全权集中于物流配送中心,包括公司工程用物资、燃器具的采购、户外管道及户内管线安装材料等。公司办公用品的采购由行政部负责。采购业务分为常备采购(预算内)、非常备采购(预算外)、政策性采购(公司战略需要)。 二、采购计划的制定和审批 公司各单位(分、子公司及各部门)每月25 日向物流配送中心报送下月的物资采购计划,物流配送中心经汇总、综合平衡后向财务部报送当月公司整体采购计划和采购资金预算。该计划应综合考虑各项物资实际库存、工程施工计划和公司资金状况。该计划亦应与各预算单位每月向财务部上报的预算报表口径一致。财务部审核通过后报总经理审批,经审批通过后下发物流配送中心执行,同时转财务部备档作为资金安排的依据。采购计划审批流程(见流程图1): 三、采购业务的执行 1、供应商的管理 (1)、采购人员应随时调查供应商的动态及产品质量。凡欲与公司建立供应关系而且符合条件者应填具“供应商调查表”(见表1),作为选择供应厂 商的参考。“供应商调查表”呈采购部经理核准后存档。 (2)、物流配送中心应在日常工作中逐步建立系统的供应商档案库,供应 商档案包括:供应商名片、供应商调查表、供应商审批表、供应商资信档案、供应
商所提供的合格证明、价格表及相关资料 (3)、每批材料供货结束后,供应商的实绩转记于“供应商资信档案” (见表2),作为评审供应商业绩的资料。 (4)、采购人员依据“供应商调查表”每半年复查一次,更正原有资料内容。供应商档案由物流配送中心的经理指派专人负责管理,未经采购部经理允许,不得随便查阅。供应商调查表应每月报一份给财务部存档。 (5)、物流配送中心应对供应商的选择建立内控审批制度。在下列情况 下,采购人员应填写“供应商审批表”(见表3),提请主管领导审批:A、原有供应商被撤点;B、开发新产品和新项目投资需引进新的供应商;C、原有供 应商由于种种原因拒绝供货需更换供应商;D、新供应商的物资更适合公司的 需求。 2、商品样品的检验: (1)、对通过初审的供应商所送检的样品,必须经质量检验部门进行各项 指标的检验和检测。 (2)、供应商提供样品同时应附带完整的自检报告及合格证明等资料。 (3)、对通过初审和提供样品检验合格的供应商方可进行供货。如果评审 结果不合格,要求供应商限期作出整改。整改后仍不合格者,取消其入选供应商资格。 3、请购申请单的处理 (1)、审核采购申请单各栏填写是否清楚,审批者是否签字。 (2)、紧急采购申请单应优先办理。
自动化专业英文简历
路飞(Monkey.D.Ruffy) political:communist Birthdate:1987-5-5 Address:Roomxxx,No.xx building,west block,Guangdong University of Technology, Higher Education Mega Center,Guangzhou Postalcode:510006 Mobile:xxxxxxxxxx E-mail:xxxxxx1@https://www.360docs.net/doc/a49295088.html, Objective Education 2006.9-2010.7 Guangdong University of Technology Major: Automation Engineering Degree: Bachelor Specialized courses:Automatic Control Theory;Theory of Circuitry;Signal& Linear System; Principle of Microcomputer; C Language; Analogical Electronics; Digital Electronics; Pro- cedure Control and Auro-Measurement Technique ; Principle of Single-Chip Computer; Power Electronics Technology; Computer Control Technology; Electric Drive Control System; Electrical and Programmable Logic Controller Applied Technology(PLC);Motor Theory and Drive;Power Supply and Distribution Technology;Datebase(SQL) and so on. Personal expertise ?Master automatic control,computer control and process control,power technology; ?Skilled in the use of PLC ,SCM,C,assembler,matlab; ?Good use of Protel ,CAD,Photoshop,Word ,powerpoint and Excel; ?Master motor control,power electronics technology;good understanding of mechanical design principles,and mechanical analysis; ?Good rational thinking ability,Optimistic attitude towards life,adaptable;with good team spirit;responsible and honest;have a harmonious interpersonal relations with others. ?Language ability:Passed CET-4 446;Master automation professional English. Project Experience And Activities ?Two week of 2009/ 5 Temperature Control System Design Project Description: Design circuits, write procedures and control the temperature of Furnace at the required temperature which allow 1 to 2 C o error with SCM;This system must has the ability of refecting disturbance. ?2008/4 Attending Physics experiment Skills Competition of GDUT Project Description:I participate in the oscilloscope Group in this competition; The main requirement of the competition is to master the operation of oscilloscope and abilities to cope with some unexpected problems 2008.6-2009.6 Guangzhou ZhouHong Educational and Cultural Development Co., Ltd. Responsibilities: Part-time;The main task is making the courseware with PPT. Hobbies Reading,singing,outdoor activities,football,basketball,watching football games fishing and so on.
100首最好听的英文歌曲
《我的名字叫伊莲》推荐,经典,流传 《Oh My Love》宁静 《Scarborough Fair(毕业生)》推荐,柔情 《better man》感慨,惊叹 《God is A Girl》推荐,经典,流传 《zombie》畅想,激烈 《Under A Violet Moon》乡村,朴实 《Canon_Flamenco》推荐,淡雅,心灵 《classicriver》伤感 《beautiful ones》摇滚,动感 《Stand》推荐,欢快 《Tears In Heaven》 《Yeah》推荐,摇滚, 《Dream Catcher》忧伤 《dragostea din tei》推荐,欢快 《Gloomy Sunday》悲伤 《El Condor Pasa (If I Could)》推荐,宁静 《Tom's Diner》推荐,说唱 《Hotel California加州旅馆》推荐,感慨,赞叹 《Eyes on me》抒情 《as long as you love me》推荐,经典 《baby one more time》推荐,经典 《right here waiting[此情可待]》推荐,经典,爱情,流传《Scarborough Fair》幽深,抒情 《i want it that way》推荐,经典 《To be with you》抒情,激昂 《Every Body》说唱,经典 《All rise》 《We Will Rock You》节奏,说唱 《蝴蝶-Le Papillon》温馨,说唱 《YELLOW》推荐,忧伤,独特 《Nothing‘s gonna change my love for you 《lonely》推荐,说唱 《Shape Of My Heart》推荐,抒情,忧伤 《nocturne》经典,曲 《as i moved on》个性,舒畅 《pretty young thing》欢快 《Traveling Light》欢快 《Seasons in the Sun》经典,抒情 《All About Us》推荐,震撼,激昂 Insatiable》邪恶,个性 《In the end》推荐,经典 《welcome to my life》 《untitled》
机械工程控制基础简答题汇总
控制论两个核心:信息和反馈 控制论与机械工程控制关系:机械工程控制论是研究控制论在机械工程中应用的一门技术学科。 控制论发展阶段及特点:第一阶段的自动控制理论,即经典伺服机构理论,成熟于40~50年代。针对工程技术运用控制论的基本原理建立起来的在复数域(频率域)内以传递函数(频率特性)概念为基础的理论体系,主要数学基础是拉普拉斯变换和傅里叶变换,主要研究单输入—单输出定常系统的分析和设计。第二阶段的自动控制理论,即形成于20世纪60年代的现代控制理论。主要以状态空间法为基础建立起来的理论体系,主要针对多输入—多输出(线性或非线性)系统研究其稳定性、可控性、可观测性等系统分析、综合以及最优控制和自适应控制等问题。第三阶段的自动控制理论,即在20世纪70年代形成的大系统理论,主要针对规模特别庞大的系统,或者特别复杂的系统,采用网络化的电子计算机进行多级递阶控制。第四阶段的自动控制理论,即始于20世纪70年代的智能控制理论。使工程系统、社会、管理与经济系统等具有人工智能。 机械工程控制论研究对象:机械工程控制论是研究以机械工程控制技术为对象的控制论问题。具体的讲,是研究在这一工程领域中广义系统的动力学问题,即研究系统在一定的外界条件(即输入与干扰)作用下,系统从某一初始状态出发,所经历的整个动态过程,也就是研究系统及其输入、输出三者之间的动态关系。 控制系统研究涉及问题分类:1)系统确定,输入已知而输出未知,要求确定系统输出并分析系统性能,此类问题为系统分析。2)系统确定,输出已知而输入未施加,要求确定输入使输出满足最佳要求,此类问题称为最优控制。3)系统已确定,输出已知而输入已知但未知时,要求识别系统输入或输入中有关信息,此类问题即滤波与预测。4)当输入与输出已知而系统结构参数未知时,要求确定系统的结构与参数,即建立系统的数学模型,此类问题即系统辨识。5)当输入与输出已知而系统尚未构建时,要求设计系统使系统在该输入条件下尽可能符合给定的最佳要求,此类问题即最优设计。 信息:在科学史上控制论与信息论第一次把一切能表达一定含义的信号、密码、情报和消息概括为信息概念,并把它列为与能量、质量相当的重要科学概念。 信息传递:所谓信息传递,是指信息在系统及过程中以某种关系动态地传递的过程。 系统:所谓系统,一般是指能完成一定任务的一些部件的组合。 控制系统:系统的可变输出如果能按照要求由参考输入或控制输入进行调节的,则称为控制系统。 控制系统组成:主要由控制装置和被控对象两部分组成。控制装置包含给定元件、测量元件、比较元件、放大元件、执行元件和校正元件,给定元件给出系统的控制指令即输入;被控对象则是看得见的实体,输出即被控量是反映被控对象工作状态的物理量。 控制系统分类:1)按微分方程分类,可分为线性系统和非线性系统,根据微分方程系数是否随时间变化,可分为定常系统和时变系统。2)按传递信号性质分为连续系统和离散系统。3)按控制信号变化规律分为恒指控制系统、程序控制系统及随动系统。4)按是否存在反馈,分为开环控制系统和闭环控制系统。 反馈:把一个系统的输出信号不断直接或经过中间变换后全部或部分的返回到输入端,再输入到系统中去。 控制系统基本要求:系统的稳定性、响应的快速性、响应的准确性。 第2章拉普拉斯变换的数学方法 复数表示方法:点表示法、向量表示法、三角函数表示法和指数表示法。 零点与极点:当s=z1, …, z m时,G(s)=0,则称z1, …, z m为G(s)的零点;当s=p1, …, p m时,G(s)=∞,则称p1, …, p m为G(s)的极点。
二、采购业务内部控制制度及控制流程
页脚内容1
为规范公司采购流程,有效降低采购成本,加强与生产、销售、财务的协同性,保证采购物资符合生产所需,杜绝超采泛采,特制订本制度。 1采购制度 1.1企业采购涉及原材料及零配件采购,临时性物品采购,办公文具用品采购、员工 食堂用品采购,固定资产采购。 1.2一切采购均需按需采购,原材料的采购计划一般由生产部门、仓储部门、财务部 门、采购部门联签;临时采购一般由需求部门提出、相关部门联签;办公文具及员工食堂用 页脚内容3
品采购一般由行政后勤部门提出,由主管部门及相关部门联签;固定资产的采购一般金额较大,纳入公司的中长期计划,联签人员级别需高于前述联签人员,重大投资一般由董事会决定; 1.3采购部门根据采购计划进行询价比,优质优价按计划采购,因市场行情发生重大变化需要对采购计划作出加采或减采调整时,一般需要特殊审批程序以确保及时有效性,有效减少重大损失或者避免错失良机。 1.4采购价格因市场的不确定性及信息的不对称,有条件的情况下应执行最高限价,并每日编制实际采购与计划采购的比较表,分析数量及价格差异的具体原因,对不良情况要及时纠正,以控制采购成本。每月再进行一次采购情况总结。 1.5采购商品入库前必须经品检部门按产品要求全方位检查,品检通过后填制产品合格确认单移交仓库仓管员点收入库并妥善保存。快递收货应当场检查验收,确保合格符合收货条件方可收货,杜绝因收货人员疏忽大意收到次品或不符合要求产品而耽误生产的现像发生。 1.6对品检质量有差异或数量有差异但仍可接受使用的产品应与供应商协商退货或折让,同意折让收货的必须经上级主管部门审批后方能收货,需要退货处理的需填制退货通知单,要分清入库暂收退货还是直接退货,直接退货程序可简化,暂收退货的单据必须妥善保存备查。对折让收货部份的折让金额单据需及时传递财务部门避免遗漏或产生漏洞。 1.7采购部、仓库需及时将订单、合同及入库单等单据及时传递到财务部门,财务部门需及时记录相关帐目。 1.8财务部需及时索取供应商对帐单及发票,并与应付帐款明细表、预付帐款明细表核实,不符的需查明原因并纠正。 页脚内容4
中英文课程描述(计算机、自动化)
课程编号:070305 Code: 070305 课程名称:单片机及应用 Name: Principle and Application of Single Chip Microcomputer (SCM) 学时:44讲授学时,12实验学时 Hours : hrs lecture, hrs lab 学分:3 Credit: ` 课程简介:本课程介绍MCS-51单片机的功能结构、工作原理、指令系统、编程技术、接口技术和实际应用。通过该课程的学习,使学生初步具备简单的单片机应用系统的设计开发能力。 Brief introduction: This course shows the introductions to functional structure, principle, instructions, assemble language programming, interface and application of MCS-51single chip microcomputer. By the study of this course, the students can get the design and development ability of simple SCM application system. 课程编号:070306 Code : 070306 课程名称:计算机原理 Name: Principle of Microcomputer 学时:72讲授学时,16实验学时 , Hours : hrs lecture, hrs lab 学分:4 Credit: 课程简介:本课程是计算机科学技术专业的一门专业基础课;该课程介绍主流微机的结构、指令及汇编语言程序设计和常用的接口电路。 Brief introduction: Principle of Microcomputer is a basic course of Computer Science and Technology; The course introduces the structure of
超好听的中英文歌100首
超好听的中英文歌100首hey Juliet lemon tree only love i still believe as long as you love me a thousand miles never had a dream come true baby one more time you are not alone disturbia because of you god is a girl sunshine in the rain just one last dance burning far away from home moonlight shadow the day you went away mirror mirror pretty boy i want it that way
can you feel the love tonight peerless save you trouble is a friend beautiful all i ever wanted rhythm of the rain anyone of us it's my life fighter hero i will always love you nobodys fool yeah tears in heaven fingers Anaesthesia Maximilian Hecker Summer Days In Bloom Maximilian Hecker end of May Keren Ann gotta have you The Weepies i remember
采购业务内部控制
第十章采购业务内部控制 一、内控目标 1促进本公司合理采购,满足公司经营需要,规范采购行为,防范采购风险; 2确保采购活动以及供应商的管理方法和程序符合国家法律、法规和本公司内部规章制度的要求; 3保证供应商的资料数据保存完整,记录真实、准确,易于管理,便于追踪,同时合理设置供应商审核程序与审核权限,提高企业的决策效益与效率; 4维护和发展良好的、长期并稳定的供应商合作关系,开发有潜质的供应商,促进企业的长远发展战略; 5确保授权合理,与采购相关的关键岗位、职责相分离,保证采购资料及数据记录的真实、准确、完整; 6加快资金周转,降低采购成本,防止资金占用,提高经营效率。 二、关键风险 1采购计划安排不合理,市场变化趋势预测不准确,造成库存短缺或积压,可能导致公司销售不及时或资源浪费; 2供应商选择不当,采购方式不合理,招投标或定价机制不科学,授权审批不规范,可能导致采购物资质次价高,出现舞弊或遭受欺诈; 3采购验收不规范,付款审核不严,可能导致采购物资、资金损失或信用受损。
三、内控范围 本手册主要描述了上海来伊份股份有限公司(以下简称本公司)关于采购管理的相关控制流程,主要包括供应商管理、食品类商品采购、非食品类物资采购、价格调整、商品生命周期管理等。 (一)购买 1.本公司的采购业务应当集中,避免多头采购或分散采购,以提高采购业务效率,降低采 购成本,堵塞管理漏洞。本公司应当对办理采购业务的人员定期进行岗位轮换。重要和技术性较强的采购业务,应当组织相关专家进行论证,实行集体决策和审批。 本公司除小额零星物资或服务外,不得安排同一部门办理采购业务全过程。 2.本公司应当建立采购申请制度,依据购买物资或接受劳务的类型,确定归口管理部门, 授予相应的请购权,明确相关部门或人员的职责权限及相应的请购和审批程序。 本公司可以根据实际需要设置专门的请购部门,对需求部门提出的采购需求进行审核,并进行归类汇总,统筹安排本公司的采购计划。 具有请购权的部门对于预算内采购项目,应当严格按照预算执行进度办理请购手续,并根据市场变化提出合理采购申请。对于超预算和预算外采购项目,应先履行预算调整程序,由具备相应审批权限的部门或人员审批后,再行办理请购手续。 3.本公司应当建立科学的供应商评估和准入制度,确定合格供应商清单,与选定的供应商 签订质量保证协议,建立供应商管理信息系统,对供应商提供物资或劳务的质量、价格、交货及时性、供货条件及其资信、经营状况等进行实时管理和综合评价,根据评价结果对供应商进行合理选择和调整。 本公司可委托具有相应资质的中介机构对供应商进行资信调查。 4.本公司应当根据市场情况和采购计划合理选择采购方式。大宗采购应当采用招标方式, 合理确定招投标的范围、标准、实施程序和评标规则;一般物资或劳务等的采购可以采用询价或定向采购的方式并签订合同协议;小额零星物资或劳务等的采购可以采用直接购买等方式。
机械工程控制基础作业
第一题:生活中常见开环控制系统与闭环控制系统综合性能分析。 电加热炉开环系统与闭环系统综合性能分析 一、反馈及反馈控制 反馈:所谓信息的反馈,就是把一个系统的输出信号不断直接地或经过中间变换后全部或部分地返回,再输入到系统中去。负反馈:如果反馈回去的信号与原系统的输入信号的方向相反,称为负反馈。正反馈:如果反馈回去的信号与原系统的输入信号的方向相同,称为正反馈。 系统中还会存在外反馈、内反馈。外反馈:在自动控制系统中,为达到某种控制目的而人为加入的反馈,称为外反馈。内反馈:在系统或过程中存在的各种自然形成的反馈,称为内反馈。它是系统内部各个元素之间相互耦合的结果。内反馈是造成机械系统存在一定的动态特性的根本原因,纷繁复杂的内反馈的存在使得机械系统变得异常复杂。 二、开环控制 开环控制是指系统的被控制量(输出量)只受控于控制作用,而对控制作用不能反施任何影响的控制方式。采用开环控制的系统称为开环控制系统。例如: 电加热炉。 被控制对象:炉子 被控制量(输出量):炉温
控制装置:开关K和电热丝,对被控制量起控制作用。 开环控制的特点: 由于开环控制的特点是控制装置只按照给定的输入信号对被控制量进行单向控制,而不对控制量进行测量并反向影响控制作用。这样,当炉温偏离希望值时,开关K的接通或断开时间不会相应改变。因此,开环控制不具有修正由于扰动(使被控制量偏离希望值的因素)而出现的被控制量与希望值之间偏差的能力,即抗干扰能力差。 开环系统主要问题:无法自动减小或消除由于扰动而产生的误差。 三、闭环控制 闭环控制是指系统的被控制量(输出量)与控制作用之间存在着反馈的控制方式。采用闭环控制的系统称为闭环控制系统或反馈控制系统。闭环控制是一切生物控制自身运动的基本规律。人本身就是一个具有高度复杂控制能力的闭环系统。 如图所示:该电热炉由于有反馈的存在,整个控制过程是闭合的,故也称为闭环控制。 可以看到:控制系统的输出量对系统的控制作用有影响,或控制器与控制对象之间既有顺向作用又有反向联系,故这种控制系统称为闭环控制系统。说明的是:输出量对系统的控制作用的影响称为“反馈”。闭环系统:控制的是控制对象的输出量 (被控量),测量的是输出量与给定值之间的偏差。因此只要出现偏差,就能自动纠偏,用它可以实现准确的控制,因此,它是自动控制系统工作的主要方式。其框图如下图所示:
采购与付款内部控制制度
采购与付款内部控制制度 1.目的 为了规范集团及下属企业的采购与付款活动的内部控制管理,明确该部分内部控制的要点,特制定本制度. 2.适用范围 2.1集团总部 2.2各二级集团、控股子公司根据本制度精神,参照各自的业务情况另行制订具体制度,报集团财务管理中心审批后执行。 3.采购与付款内部控制目标 3.1保证与其他业务活动的要求一致。采购与付款活动包括定货要求的提出与审批、供应商的选择与报价、商品的运输与验收、款项的支付等,必须按照单位实际业务需要进行。 3.2保证会计核算资料的合法性、真实性、完整性和及时性。 3.3保证支付款项获得相应的商品和劳务 3.4保证账款按期归还,维护单位对外信誉。 4.采购与付款控制的主要凭证和记录 4.1单位应在采购与付款环节设置相关记录、填制相应凭证、建立完整的采购登记制度,加强请购手续、采购定单或合同、验收证明、入库凭证、采购发票等凭证和记录的相互核对工作。 4.2凭证和记录的设计和编制要求: 4.2.1特定的凭证必须由特定岗位的特定人员编制、其他人员不可替代。 4.2.2有效的凭证至少需要经过两人、或两人以上的部门和人员履行必要手续。 4.2.3凭证的填制与相应的业务操作应该结合起来,但应与业务的授权严格分离。 4.3.3凭证的传递可以通过套写多联的方式进行。
4.3.4凡有条件编号的凭证必须编号。 4.4采购与付款业务所涉及的有效的凭证主要有以下几种: 4.4.1请购单:是由使用部门或领用部有关人员填写并部门负责人签字和单位相关领导核准后送交采购部门订货、催办、直到收到货物为止的业务核准通知凭证。不便编号。 4.4.2订购单或采购合同:是由采购部门填写,购销双方应该共同遵守的一种契约。应预先编号并经被授权人员签字。副本应送达验收、财务部和仓储部门,作为接收货物并与发票核对的依据。 4.4.3验收单:是验收部门对商品到达或劳务完成时进行验收、检验所编制反映验收意见和收货部门或接受劳务部门接收责任的连续编号的凭证。验收单副联应送达采购部门、财务部门和接收部门。 4.4.4入库单:是仓库部门编制反映入库保管责任的连续编号的凭证。副联送财务部门。(可与验收单合并使用) 4.4.5卖方发票。 4.4.6付款申请单。 4.4.7业务台账。 4.4.8财务帐证:转帐凭证、付款凭证、材料采购明细帐、应付账款明细帐、现金银行存款日记帐。 4.4.9卖方对账单。 5.采购与付款控制的不相容职务 5.1请购与审批 采购申请必须由业务、行政、仓库等部门提出,批准请购单的人员不得兼任请购业务,具体采购工作由采购部门完成。 5.2询价与确定供应商 采购询价人员没有对供应商选择的决定权 5.3采购合同的订立和审计 采购合同的拟订、谈判和订立应当与合同的审批、审计相分离 5.4采购与验收 采购人员不能同时兼任采购物品或劳务的验收工作。
自动化岗位面试求职英文自我介绍范文
提供专业的word版文档,优质的服务,希望对您有帮助/双击去除 自动化岗位面试求职英文自我介绍范文generalIntroduction* IamathirdyearmastermajorinautomationatshanghaiJiaoT onguniversity,p.r.china. educationbackground In1995,IenteredtheNanjinguniversityofscience&Techno logy(NusT)--widelyconsideredoneofthechina’sbestengineeringschools.Duringthefollowingundergrad uatestudy,myacademicrecordskeptdistinguishedamongth ewholedepartment.IwasgrantedFirstclassprizeeverysem ester,andmyoverallgpA(89.5/100)rankedNo.1among113st udents.In1999,Igottheprivilegetoenterthegraduatepro
gramwaivedoftheadmissiontest.IselectedtheshanghaiJi aoTonguniversitytocontinuemystudyforitsbestreputati ononcombinatorialoptimizationandNetworkschedulingwh eremyresearchinterestlies. Attheperiodofmygraduatestudy,myoverallgpA(3.77/4.0) rankedtop5%inthedepartment.Inthesecondsemester,Ibec ameteacherassistantthatisgiventotalentedandmatureds tudentsonly.Thisyear,IwontheAcerscholarshipastheone andonlycandidateinmydepartment,whichistheultimateac coladefordistinguishedstudentsendowedbymyuniversity .presently,Iampreparingmygraduationthesisandtryingf orthehonorofexcellentgraduationThesis. researchexperienceandacademicactivity whenasophomore,IjoinedtheAssociationofAIenthusiasta ndbegantonarrowdownmyinterestformyfutureresearch.In 1997,Iparticipatedinsimulationtooldevelopmentforthe schedulingsysteminprof.wang’
100首英文经典歌曲
131.bryan adams--here i am https://www.360docs.net/doc/a49295088.html,/mp3/13.mp3这首真的很不错,你听听,不好撞墙1.gregorian--so sad https://www.360docs.net/doc/a49295088.html,/hard7/user3/7003594/mydisk/sosad.mp3 2.george michael--jesus to a child https://www.360docs.net/doc/a49295088.html,/0/332/hiwarrior/sound/200599215354384.mp3 3.the corrs--only when i sleep https://www.360docs.net/doc/a49295088.html,/blogmusic/only.mp3 4.guns n' roses --don't cry https://www.360docs.net/doc/a49295088.html,/f/guns_and_roses_-_don_t_cry.mp3 5.sade --no ordinary love https://www.360docs.net/doc/a49295088.html,/music/Sade%20-%20No%20ordinay%20love.mp3 6.secret garden--- nocturne https://www.360docs.net/doc/a49295088.html,:82/52yingyin/sanwu/nocturne.mp3 7.iris - goo goo dolls http://members.shaw.ca/tff6/Goo Goo Dolls - Iris.mp3 8.sinead o'connor--- you made me the thief of your heart https://www.360docs.net/doc/a49295088.html,/mp3/ymmttoyh.mp3 9.michael bolton-- said i loved you but i lied https://www.360docs.net/doc/a49295088.html,/m/music/silybil.mp3 10.the cranberries-- zombie https://www.360docs.net/doc/a49295088.html,/music_2/g/296/4.Wma 11.martika--- toy soldiers https://www.360docs.net/doc/a49295088.html,/musicfile//t9t8cn/2005-3-21/03/17.Wma 12.annie lennox-- why 13.glenn frey--the one you love https://www.360docs.net/doc/a49295088.html,/en/va/Always/13.Wma 14.enya--how can i keep from singing http://222.92.21.147/sudaceo/upload/How-can-I-keep-from-singing.mp3 15.王菲--eyes on me https://www.360docs.net/doc/a49295088.html,/00data/Eyes_on_Me.mp3 16.all 4 one-- i swear https://www.360docs.net/doc/a49295088.html,/6220396/sosad.mp3 17.emilla--big big world https://www.360docs.net/doc/a49295088.html,/images/sub/edu/english/music/big.mp3 18.shawn colvin--sunny came home https://www.360docs.net/doc/a49295088.html,/Sunny.mp3 19.sting ----- shape of my heart https://www.360docs.net/doc/a49295088.html,/mp3/Shape_Of_My_Heart.mp3 20.timo tolkki—are you the one https://www.360docs.net/doc/a49295088.html,/UploadFile/2005-8/200582219594682862.mp3 21.caron nightingale--- promise don't come easy
