Energetic Derivatives Of Tetrazole

Introduction
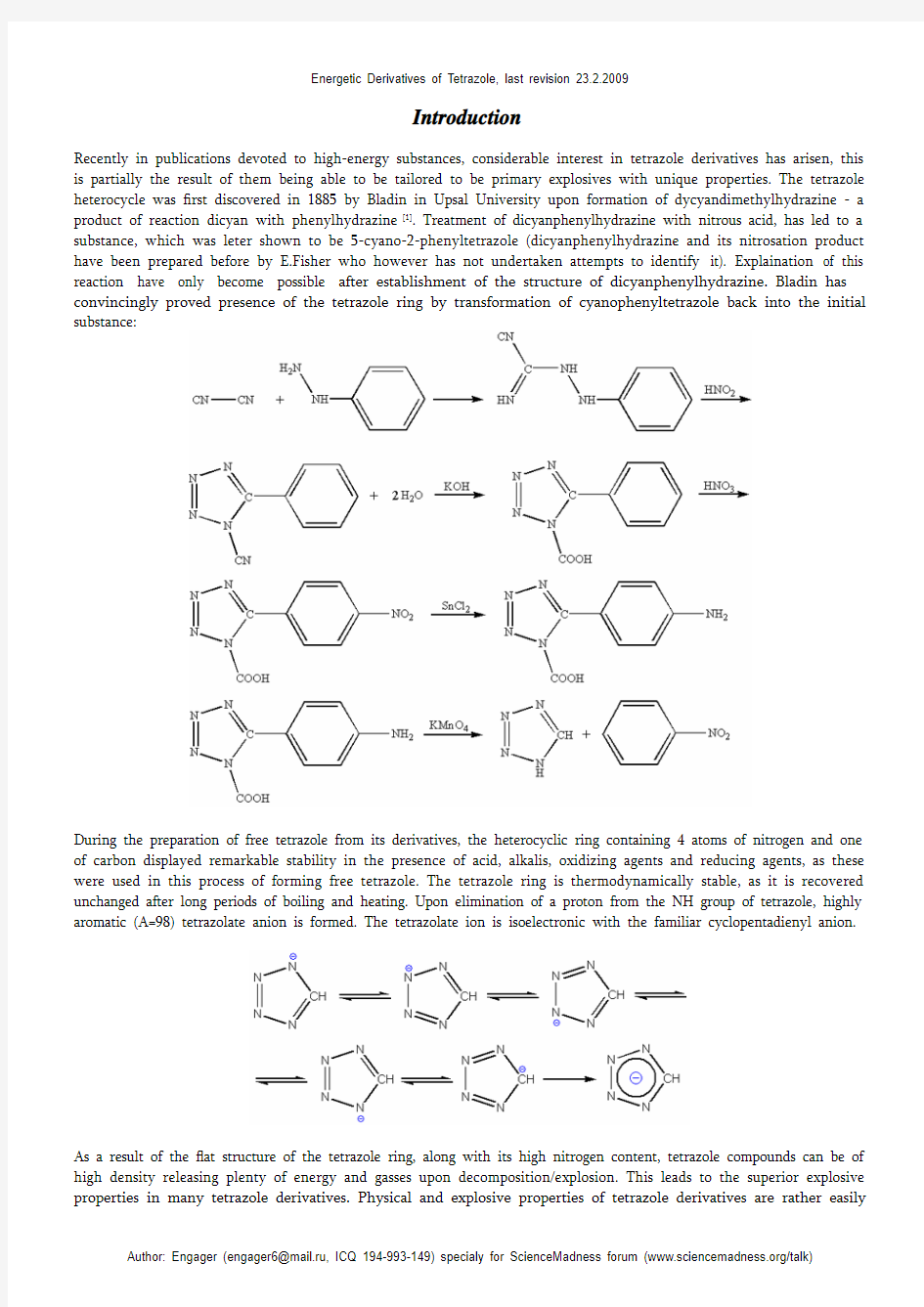
Recently in publications devoted to high-energy substances, considerable interest in tetrazole derivatives has arisen, this is partially the result of them being able to be tailored to be primary explosives with unique properties. The tetrazole heterocycle was first discovered in 1885 by Bladin in Upsal University upon formation of dycyandimethylhydrazine - a product of reaction dicyan with phenylhydrazine [1]. Treatment of dicyanphenylhydrazine with nitrous acid, has led to a substance, which was leter shown to be 5-cyano-2-phenyltetrazole (dicyanphenylhydrazine and its nitrosation product have been prepared before by E.Fisher who however has not undertaken attempts to identify it). Explaination of this reaction have only become possible after establishment of the structure of dicyanphenylhydrazine. Bladin has convincingly proved presence of the tetrazole ring by transformation of cyanophenyltetrazole back into the initial substance:
During the preparation of free tetrazole from its derivatives, the heterocyclic ring containing 4 atoms of nitrogen and one of carbon displayed remarkable stability in the presence of acid, alkalis, oxidizing agents and reducing agents, as these were used in this process of forming free tetrazole. The tetrazole ring is thermodynamically stable, as it is recovered unchanged after long periods of boiling and heating. Upon elimination of a proton from the NH group of tetrazole, highly aromatic (A=98) tetrazolate anion is formed. The tetrazolate ion is isoelectronic with the familiar cyclopentadienyl anion.
As a result of the flat structure of the tetrazole ring, along with its high nitrogen content, tetrazole compounds can be of high density releasing plenty of energy and gasses upon decomposition/explosion. This leads to the superior explosive properties in many tetrazole derivatives. Physical and explosive properties of tetrazole derivatives are rather easily
modified by replacement of substituents on the tetrazole ring with various functional groups. The combination of interesting energetic properties and unusual chemical structure and draws numerous researchers to this unique class of compounds.
Many tetrazole based explosives can employed as primary explosives, sensitizers or components of electric igniters. The most widespread derivative in use is 5-aminotetrazole which is more easy to produce than unsubstituted tetrazole, and is often used as a starting material for the synthesis of other tetrazole derivatives. Oxidation of amino groups in 5- aminotetrazole leads to 5,5 '-azotetrazoles which can be applied as primary explosives or as components of electric igniter mixtures. Condensation of 5-aminotetrazole ring with aminoguanidine results in the known sensitizer tetracene, widely used in initiating mixtures. Diazotization of the amino group in 5-aminotetrazole results in the formation of highly reactive(and explosive) tetrazole-diazonium salts, which are the starting product for many interesting tetrazole derivatives. Condensation of the diazonium salt of tetrazole with 5-aminotetrazole results in salts of diazoaminotetrazole; some have been suggested for use in primary explosive mixtures and compositions for electric igniters. Substitution of the diazo group to the nitro group by the Sandmeyer Reaction results in 5-nitrotetrazole; the basis of the newest class of highly effective primary explosives. Some tetrazole derivatives maintain record values of nitrogen content, for example action of hydrazine chloride on diazotetrazole leads to formation of 5,5’-bis-diazotetrazolehydrazide, containing about 90 mass % of nitrogen, and the action of nitrous acid on 5-tetrazylhydrazine leads to the formation of 5-tetrazylazide, which is one of the richest on nitrogen from all known compounds. Tetrazylazide forms numerous salts, many possessing powerful initiating abilities, and incredible brisance, but are extremely sensitive to external influences. Some researchers have reported that the sensitivity of the tetrazylazides can be reduced in organic derivatives.
In the subsequent sections these substances and their derivatives will be considered in more detail. At once it would be desirable to note that despite of significant theoretical and practical interest many of these substances, they are investigated badly enough and frequently the trustworthy information about them has only the general character. It is necessary to remember that the highly energetic derivatives of tetrazole always represent some extent of danger, and to work with them demands extensive care and accuracy, as well as knowledge in the field of organic chemistry and experience in working with sensitive explosive materials. The purpose of the given manual is only to supply the beginner researcher with the known facts and to combine the accessible information. Working with these substances you always have to go inside an area of organic chemistry and of explosives which to some extent is novel and has not been
completely thoroughly studied. Your actions should be guided by your logic combined with knowledge and experience in similar areas. The authors reserve the rights to not carry the responsibility for any possible negative consequences of practical use of the given materials.
General properties of tetrazoles [2]
Tetrazoles are characterized by five atom ring with two unsaturated bonds, consisting of 4 atoms of nitrogen and 1 atom of carbon. Numbering of the atoms starts from the nitrogen next to the carbon, it has first number, next nitrogen is second, and so on. Tetrazole cycle exists in two tautomeric forms, differing by position of hydrogen atom, to identify one of the isomers isomers, the name of the tetrazole derivative is supplied with acid hydrogen atom position.
On the basis of the tautomeric formulae shown for 5-monosubstituted tetrazoles (where R is any substituent) there are three classes of monosubstituted and two classes of disubstituted deriva?tives (where the H is replaced). Also, there are fused ring tetrazoles of the 1,5-disubstituted class. In addition, there are salts and substituted derivatives which have 2,3-, 1,4- and 1,2-substituents. However, compounds of ordinary interest are usually 1,5-, 2,5-or 1,3-substituted. Bis explosive compounds may be connected at the 5,5'-positions. Bis explosive salts may be connected at either the 1,1'- or the 5,5'- position with the metallic anion.
The majority of tetrazoles are crystaline solids. There is considerable variation in thermal stabil?ity, viz, derivatives which melt above 150°C do so with decomposition, while 5-guanylaminotetrazole does not melt at 300°C. In general, most of the tetrazoles are acids and often yield explosive salts. Tetrazoles are generally soluble in polar solvents, and insoluble in non polar solvents, 1-H tetrazole has good solubility in water. Unsubstituted tetrazole and C-substituted 1-H tetrazoles show amphoteric properties, they are weak NH-acids (for 1-H tetrazole рКа = 2,68) and readily form salts with alkaline and alkali-earth metals, being a weak organic bases tetrazoles form salts with strong mineral acids. With ions of transition metals tetrazoles often form coordination complex compounds through N-2 atom, generally acting as monodentante ligands. Tetrazole and it’s derivatives enter electophilic and nucleophilic substitution reactions generaly on the 5 ring position. Salts of C-substituted 1-H tetrazoles upon alkylation by alkylhalogenides, esters of sulphuric acid or organic acids, or diazomethane, produce mixtures of 1,5- and 2,5- substituted products, because of ambidentate nature of tetrazolate anion. Disubstituted tetrazoles on further alkylation form tertiary salts on N-4 atoms. Thermal destruction of tetrazole cycle usually take place at 150-200°C. The tetrazole ring can also be broken by action of strong acid while heating to 160°C, or by the action of strong aqueous solutions of bases, C-subtituted 1-H tetrazoles are generaly stable at this conditions. It is stable to strong oxidizers, but powerful reducers such as lithium-aluminium hydride (LiAlH4) upon reaction with 1,5-disubsitituted 1-H tetrazoles, causes the ring to rupture forming secondary amines with release of hydrogen azide. The tetrazoles, in general, can be looked upon as gas generators, useful where instantaneous or progressive pres?sure effects are required. They possess moderate brisance. However, their salts, which may de?tonate with extreme brisance, can be used as primary explosives, as can 1- and 2-Methyl-5-nitro tetrazoles or other 5-nitrotetrazole derivatives, or tetrazylazide derivatives.
Systematization of data on about twelve 5-substituted tetrazoles allowed Bates and Jenking to make the conclusion that explosive properties of tetrazole are closely related to their structure. Bates and Jenkins have suggested that the ranking in explosive behavior of 5-substituted tetrazoles could be related to the substituent's electron withdrawing power: the more electron withdrawing, the more explosive is corresponding compound. The ranking observed for the substituent in the 5 position ranged from mild ignitions for 5-methyl tetrazole to instability in the following order: CH3 = C6H5 (mild ignitions) < NH2 < H< NHNO2 (componds exploded) < bistetrazole < 5,5'-azoditetrazole (do not detonate RDX), < Cl < NO2 (very powerful expls) < N3 (very sensitive) < N2+ (unstable). The salts of 5-substituted tetrazoles could also be ranked according to their sensitiveness. The salts of Ag, Hg, and Pb gave more sensitive compounds and greater initiating
power than the alkali metal salts. The same salts of 5-chlorotetrazole were too sensitive and corrosive, and those of 5-azidotetrazole were too sensitive for handling.
References:
1. R.C. Elderfield “Heterocyclic Compounds” Volume 8, New York, 1961, pp 8-11
2. Basil T. Fedoroff & Oliver E. Sheffield “Encyclopedia of explosives and related items” Volume 9, T111-T112 .
5-Aminotetrazole and derivatives
5-Aminotetrazole was first prepared by Thiele, by diazotation of aminoguanidine with sodium nitrite in hydrochloric acid environment. 5-Aminotetrazole crystallizes from water solution in the form of the monohydrate, which are colorless prisms or leaflets, losing water above 100°С and melting with decomposition at 200-203°С. 5-Aminotetrazole is badly soluble in alcohol and more readily in ether. It is also soluble in water solutions of bases and strong acids, and has good solubility in hot and poor solubility in cold water. 5-aminotetrazole's heat of combustion is 246.2 kcal/mol, standard enthalpy of formation is -49.7 kcal/mol. 5-Aminotetrazole shows weak acid properties, and in the anhydrous state is extremely hygroscopic. It is stable to heat, and its dissociation constant is about 1*10-4. Besides its acid properties, upon reaction with strong mineral acids 5-aminotetrazole can act as base, as can many organic amines. In general 5-aminotetrazole acts as an amphoteric substance, with behavior similar to that of amino acids. [3,4]
5-Aminotetrazole draws itself attention as high nitrogen substance for its use as components of rocket propellants, explosive mixtures and as starting material for synthesis of other tetrazole derivatives. The high chemical stability of the tetrazole ring in addition to fact that substituents on the tetrazole ring are usually entered during ring formation, leaves the amino group as the only reasonable target for chemical manipulations. The amino group of 5-aminotetrazole has all the common properties of that functional group, and can be related in chemical behavior to the amino group of aniline.
5-Aminotetrazole forms salts with metallic cations, some of them are explosive. The cobalt salt Co(CN5H2)2?XH2O, exists as pink water soluble crystals which explode upon heating to 228°С. The nickel salt Ni(CN5H2)2?H2O exists as blue water soluble crystals which explode upon heating at 290°С. The lead salt Pb(CN5H2)2 , exists as colorless crystals which deflagrate upon heating to 303°С. The mercury salt Hg(CN5H2)2 exists as white water insoluble crystals, they explode when dropped on a hot plate heated to 256°С. The Hg salt's shock sensitivity in the lead weight test is 50% explosions using a 2.5 kg weight and 38 cm drop height. The copper salt Сu(CN5H2)2?H2O exists as green very slightly water soluble crystals. A 2.5 kg weight with an drop height of 68 cm gives 50% of explosions when the copper salt is tested and it deflagrates when heated to 164°С. Aminotetrazole in the presence of copper sulfate and sodium acetate produces a green amorphous precipitate, which can be used as a diagnostic test for aminotetrazole. This precipitate is insoluble in acetic acid, and is soluble in hydrochloric acid. [3,4]
Treatment of 5-aminotetrazole with sodium nitrite in hydrochloric acid was attempted by Thiele. It resulted in formation of compound which was not identified because it exploded in solution at 0°С. Later it was shown, than this reaction results in formation of extremely unstable and sensitive diazotetrazole, which can spontaneously detonate in solution as weak as 1%.[1,2] However the formation of the diazonium compound is very useful in the synthesis of other 5-substituted tetrazoles, such as the nitro, bu the Sandmeyer Reaction. 5-aminotetrazole is not explosive by itself, its use is as a starting product for synthesis of many explosive derivatives such as 5,5’-azotetrazole, 5-nitrotetrazole, bis-diazotetrazylhydrazine, 5-nitraminotetrazole, 5-chlorotetrazole, and others. The high safety and stability of 5-aminotetrazole allows its preparation on any desired quantity; in the lab or mass industrial production. When not being used for the synthesis of other tetrazoles, this high nitrogen compound fins use as cooling agent in rocket propellants, where it produces almost smokeless burning without any impairment of ballistic potential.
There are many different ways to produce 5-aminotetrazole, although among them there are only two methods of practical interest. By the Thiele method 5-aminotetrazole is produced by the action of nitrous acid on aminoguanidine nitrate. Later Ganch showed doubt of Thiele's views and proved that the product of this reaction is guanylazide, which on boiling in presence of a base cyclizes to 5-aminotetrazole[2]:
The cyclization of guanylazide is also catalyzed by acid. Ganch and Fort showed that boiling of guanylazide nitrate in water for 2 hours also results in formation of 5-aminotetrazole, without release of hydrogen azide. Later Shtolle and Shick are succeeded in the synthesis of 5-aminotetrazole by action of hydrogen azide on dicyandiamide (cyanoguanidine). A detailed look at the reaction mechanism has revealed that dicyandiamide first forms 2 molecules of cyanamide, which react with hydrogen azide forming guanylazide, which is later cyclized to 5-aminotetrazole. Reaction with free cyanamide proceeds in the same way[2]:
Both methods give good results, but also have advantages against each other. Advantage of Thiele method is speed and safety – whole process takes time about several hours, but yield is 75%(I got 82% yield, and microtek has cited 99% yield). Shtolle method has the advantage of higher overall yield and slightly better product quality, but is very time consuming (process takes time about several weeks) and forces working with dangerous, highly toxic, and explosive hydrogen azide. Both methods yield 5-aminotetrazole pure enough for any potential use. Synthesis with both methods are shown bellow: Thiele method[1]. 34g (0.25 mol) of aminoguanidine bicarbonate is added to 217 ml of 15% nitric acid (0.561 mol), and mixed until evolution of carbon dioxide is stopped and resulted aminoguanidine nitrate is fully dissolved in solution. Yellow transparent solution is diazotized by slow addition of 17.2g sodium nitrite (0.25 mol) in 35 ml of water. Addition is accompanied by stirring, and temperature buring all addition period is kept between 20-25°С by using water bath if needed (note #1). After completion of reaction the diazotation mixture is allowed to sit for 20 minutes at room temperature, and 29g of sodium carbonate is added (or 46g of sodium bicarbonate). Mixture is then heated on a waterbath and refluxed for 4 hours. The solution is then neutralized by 30% sulphuric acid to pH=4, cooled to room temperature and allowed to sit over night (note#2). The precipitated crystals of 5-aminotetrazole monohydrate are filtered, washed with cold water and dried. Yield is about 70-74% based on aminoguanidine.
Notes:
1. Diazotation proceeds smoothly with little exotherm. If reaction mixtures begins to foam (this is the result of decomposition of nitrous acid), mixture must be stirred until form is settled before adding new a portion of nitrite solution. If reaction is carried put in the right way, it takes time about 10-15 minutes and proceeds with negligible evolution of nitrogen oxides.
2. 5-Aminotetrazole has an affinity to form supersaturated solutions. Often it does not begin crystallization even after full cooling, in that case crystallization should be assisted by introduction of a seed crystal, or by rigorous friction of a glass rod on side wall of reaction vessel (below liquid). Crystallization is generally fully complete after 12 hours from start.
Schtolle method[2]: 84g (1 mol) of dicyandiamide and 130g (2 mol) of sodium azide are dissolved in 1 liter of water at 50°С, then 172 ml of 36% hydrochloric acid is added slowly with shaking in small portions (note #1). Mixture is allowed to sit for 4-5 days, precipitated 5-aminotetrazole monohydrate is filtered off, washed with cold water and dried. Yield can be up to 97% (note №2).
Notes:
1. Reaction proceeds with release of highly volatile and highly toxic hydrogen azide, which causes severe headaches and decomposition erythrocytes in the bloodstreamI inhalation of fumes in high concentration can cause death because of paralysis of breath center. Work should be carried out in an efficient hood or outdoors. Drying wet product must also be done with efficient ventilation as a result of residual hydrogen azide.
2. After noted time crystallization may not start (see note#2 in Thiele method). Product yield strongly depends on sitting time, literature sources show that it can reach 97% of theory upon very long standing, however after 5 days the yield is only about 65% of theoretical. Yield is also dependant on minimizing evaporation of hydrogen azide, so for maximum yields it is beneficial to carry out the reaction in a sealed vessel to minimize evaporation.
Additional information
1. 5-Aminotetrazole nitrate[3]. Compound is a simple salt of 5-aminotetrazole, which forms a cation through the aminogroup, in analogous manner to ammonia forming ammonium salts. It is a colorless crystalline substance which melts with explosion at 174°С. Heat of combustion is measured at 224.1 kCal/mol. Solutions of 5-aminotetrazole nitrate show acidic pH as a result of hydrolysis and the compound decomposes in base environment. It can be simply made by crystallization of a hot solution of 10g anhydrous 5-aminotetrazole in 16 ml concentrated niric acid and 15 ml of water[5]. Prolonged heating leads to decomposition. The crude product, wet with nitric acid is recrystallized from water, filtered and dried. Yield is about 15g of pure product (97%).
2. Double salt of 5-aminotetrazole with silver perchlorate Ag(N4C)NH2?AgClO4. This compound has been proposed for use as thermally stable primary explosive[6]. Salt is powerfull enough to initiate TNT and hexanitrostilbene, and was shown to be thermally stable during heating for 50 hours at 260°С. It is easily ignited by an electic wire and it's preparation is given in patent US3663553: A solution of 8.2g 5-aminotetrazole in 184 ml of 70% perchloric acid is added with stirring to solution of 16.6g of silver perchlorate in 32 ml of water. Mixture is stirred for 30 minutes, 380 ml of water is added and stirring is continued for another 30 minutes. Precipitate is filtered off, washed with water, then isopropyl alcohol and dried at 150°С for 5 hours.
3. 5-Nitraminotetrazole H(CN4)NHNO2. Exists as colorless crystals, exploding with an orange flash when heated to 140°С. Heat of decomposition is 2.63 MJ/kg, and its standard heat of formation is -944 kJ/mol, calculated density and detonation velocity are 2.06 g/cm3 and 9130 m/sec respectively, however these values are probably overestimated[7]. It is extremely sensitive to shock and friction and has good solubility in water, ether and dioxane, poor solubility in benzene. Forms hydrates. Exists as a double basic acid, strong by first stage, dissociation constants pKa = 2.5 and 6.1 respectively. Initially it was thought that the acidic hydrogen atom was situated in the nitramine group, but now it is proved that acidic protons are situated on the tetrazole ring, and one of them forms an intramolecular hydrogen bond, so substance can be more correctly called 5-nitriminotetrazole. 5-Nitraminotetrazole forms stable salts, many of which are explosive. Potassium salt KCHN6O2 exists as almost colorless plates, exploding with aviolet flash when dropped on a hotplate heated to 220°С. The diammonium salt (NH4)2CN6O2 exists as short colorless needles with a melting point of 220°С. The silver salt AgCHN6O2 is an amorphous white powder, insoluble in water, deflagrates on heating.
5-Nitraminotetrazole synthesis[5]: 14.8g of 5-aminotetrazole nitrate is with stirring and cooling, added to 20 ml of concentrated sulphuric acid. Mixture is allowed to sit at room temperature until it becomes homogeneous then 250 ml of ice and water is added and the whole mixture is then neutralized with barium carbonate. The mixture is heated on a water bath until carbon dioxide evolution stops and it is filtered to remove the insoluble barium sulphate precipitate. The precipitate is then washed with few portions of water, and combined filtrate is evaporated under reduced pressure to volume of about 100 ml. Product is extracted with 5 portions of 100 ml of ether and extract is evaporated almost to dryness and then 250 ml benzene is added. 5-Nitraminotetrazole precipitates as colorless plates, yield is about 57%. Diammonium salt synthesis: 127.5g of 5-aminotetrazole monohydrate is added with stirring to 300 ml of concentrated sulphuric acid heated to 40°С, mixture is stirred until 5-aminotetrazole dissolves, and then cooled to 20°С. 120 ml of 90% nitric acid is added drop by drop with good stirring, keeping temperature in the 20-25C range by means of ice bath. When addition is finished the mixture is then taken out of the ice bath and allowed to sit for 15 minutes at room temperature after which it is and poured onto 3 kg of crushed ice, and neutralized by 25% ammonia solution to pH=5. Reaction mixture is allowed to sit for a night at 0-5°С during which time crystals of the diammonium salt precipitate. The 5-nitraminotetrazole is filtered and dried.
References:
1. P.F. Bubnov “Primary Explosives and Initiation Devices” part 1, Moscow, 1940, pp 309-311.
2. L.I. Bagal “Chemistry And Technology of Primary Explosives”, Moscow, 1975, pp 394-395.
3. Basil T. Fedoroff & Oliver E. Sheffield “Encyclopedia of explosives and related items” Volume 1, A258-A259.
4. Basil T. Fedoroff & Oliver E. Sheffield “Encyclopedia of explosives and related items” Volume 9, T116-T117.
5. Journal of Organic Chemistry, 18-8, pp 941 – 945 ; R.M.Herbst, J.A.Garrison “The Nitration Of 5-Aminotetrazole”.
6. US Patent №3663553, Disilver aminotetrazole perchlorate, patented 16 may 1972.
7. T.M.Klapotke “High Energy Density Materials”, Springer, pp 99-113.
Some photos for this chapter
Description: Two photos at the top show, 5-aminotetrazole crystalls floating in mother liquer obtained by Thiele method, at lower left crystalls are dried on the air, lower right photo shows 5-ATZ obtained by Schtolle method.
Description: This two photos are small lustrous needle like crystalls of ammonium 5-nitraminotetrazole, made by nitration of 5-aminotetrazole with sulphuric/nitric acid mixture and allowing solution to sit for a night.
5-Diazotetrazole and derivatives
Diazotetrazole was first prepared by Thiele during the diazotation of 5-aminotetrazole with sodium nitrite in hydrochloric acid, but he failed to indentify reaction product, because at 0°С it exploded in solution. Later it was shown that the product of this reaction is the extremely unstable diazotetrazole. Diazotetrazole is an extremely sensitive and unstable explosive, 6-7% solutions can explode spontaneously even at 0°С, breaking the glassware used for the diazotation. In acidic environment diazotetrazole forms ions of tetrazolediazonum, which are combined with acid anion to form the diazonium salt. Tetrazole diazonium salt is also very unstable and its behavior resembles that of free diazotetrazole. In a basic environment diazotetrazole forms anions of tetrozoliumdiazotate, which are combined with base cations to form stable salts, readily soluble in water, and explosive in the dry state[1].
The extremely unstable nature if diazotetrazole and it’s diazonium salts is almost unprecedented for known chemical compounds, it can explode even in 1% solutions. The most likely reason for this instability is the strong electron withdrawing effect of the diazogroup, which lowers the stability of tetrazole ring and lowers the activation energy for decomposition to a neglible level. Stability of tetrazoliumdiazotate salts can be explained by the electron saturation of diazogroup by hydroxide ions, oxygen atom on the end of a diazogroup lowers its electron withdrawing ability and stabilizes the tetrazole ring. Tetrazolediazotate salts are so stable, what they can be simply prepared in free state. Tetrazoliumdiazotate can exist in two isomeric forms; cis and trans form. Usualy the unstable cis form is formed first, and transforms to stable trans form by long boiling, reverse transformation takes place only by action of ultraviolet radiation:
The difference in stability of two forms is so great, that they can be practically be termed as different substances, because of the large difference in physical properties. For example the pale yellow or colorless trans diazotate salts are quite stable in the dry state, but the cis diazotate salts are unstable and explosive in dry state even with stabilizing aromatic rings. During acidification the bifunctional trans-diazotate anions transform to trans-diazotetrazole hydroxide or to N-nitrosoamine, which are further transformed to the cation of tetrazolediazonium. Formation of nitrosoamine can be easily noted by green coloration of solution, usual for nitrosoamines. Freshly prepared diazotetrazole solutions are always slightly green colored.
Despite the instability of tetrazolediazonium cations, they have sugnificant practical importance in the chemical reactions of the aminogroup in 5-aminotetrazole. The tetrazolediazonium cation is very reactive and readily loses nitrogen, that allows for replacement of the diazo group with many other funtional groups. This replacement takes place during Sandmeyer reaction – replacement of diazogroup by halogen or pseudohalogen in presence of cupric salts, this reaction was first discovered in 1884 by T. Sandmeer and now it is very important in many fields of organic synthesis. The reaction scheme and mechanism are shown on picture below:
The reaction also takes place in the presence of cooper (II) salts or in the presence of copper powder (Gatterman reaction). Because of the extreme instability of diazotetrazole, diazotation and replacement of diazogroup are usually made simultaneously, in conditions optimized for maximally fast transformation of tetrazolediazonium cation to final product. This can be achieved by an excess of cupric salt catalyst and usage of reverse diazotation, conditions by slow addition of an acidified solution of 5-amino tetrazole to copper salt/nitrite mixture. The reaction is carried out with strict temperature control, which is essential for the stability of the diazonium salt, while allowing for a maximum rate of diazo group replacement (15-18°С Resultant copper salt of the desired 5-substituted tetrazoles are usually insoluble in cold water and precipitate from the reaction mixture. Side reactions also take place, resulting in the formation of 5,5’-azotetrazole, 5,5’-bistetrazole and 5-hydroxytetrazole, as impurities in reaction product.
Another important reaction of diazotetrazole is additon to electron donating moieties. For example the action of a slightly acidic solution of sodium nitrite on 5-aminotetrazole results in formation of tetrazolediazonium salt, which is further condensed with unreacted 5-aminotetrazole, forming 5,5’-diazoaminotetrazole. Salts of tetrazolium diazotate in fairly concentrated solultion on prolonged boiling or action of a stream of carbon dioxide, are transformed into extremely explosive salts of hydroxyazotetrazole; the mechanism of this reaction is unknown.
The Sodium salt of trans-tetrazolium diazotate Na(N4C)-N=N-ONa, exists as white needle-like crystals, is very soluble in water, and weakly soluble in ethanol[1]. On heating the salt deflagrates without melting, solutions of the salt is acidic due to the hydrolysis. In a neutral or weakly acidic environment sodium salt solution reacts with lead salts, forming the lead salt of oxyazotetrazole, insoluble in water, alcohol and either, but soluble in hydrochloric acid, sodium hydroxide and ammonia. Salt deflagrates at 360°С. Barium salt of tetrazoliumdiazotate BaCON6 – yellow crystalls, readily soluble in water[1].
Diazotetrazole synthesis[2]: 5g of aminotetrazole is dissolved in 30 ml of water, then 6 ml of 25% sodium hydroxide and 3.4g of sodium nitrite are added. After the nitrite is completely dissolved the mixture is cooled in an ice bath, placed in dropping funnel and is added slowly in 10-12 minutes with efficient stirring and cooling by ice cold water to mixture of 16 ml of 30% HCl with 170g of ice. Temperature of the reaction mixture should be below or at 0°С all times (Note №1). In the end of reaction mixture has slightly green color, because of presence of equlibrium quantities of nitrosoamine. Diazotetrazole solution is best used imidately, if that is imposible, it should be imidately transformed to tetrazolium diazotate by treatment with solution of sodium hyroxide (Note №2). Reaction scheme is shown below:
Hazard notes:
1. Diazotetrazole is an extremely sensitive explosive, it’s water solutions at concentrations about 6-7% explode spontaneously even at 0°С, breaking up glassware used for diazotation. According to some sources at 0°С even 2% solutions can explode.
2. Because of the extreme unstable nature of diazotetrazole, it’s usually produced in the form of 2-3% solutions with use of appropriate safety procedures (use of shields), and if it is not needed for further synthesis, diazotetrazole must be imidately transformed into tetrazolium diazotate salt by action of 25 ml of 25% sodium hydroxide (until basic reaction). If diazotetrazole is needed for further synthesis, the solution of tetrazolium diazotate salt is cooled to 0°С and is activated by addition of corresponding amount of hydrochloric acid.
Additional information
1. Sodium salt of oxyazotetrazole Na2C2N10O?5H2O. Then sodium tetrazolium diazotate solution in fairly concentrated solution is boiled for long time, or it’s warm solution is treated with stream of CO2, sodium salt of diazotetrazole trans-forms to sodium salt of oxyazotetrazole, precipitated from solution because of moderate solubility in water[1]. Salt can be easily differed from diazotate by its characteristic dark-yellow color. Mechanism of this reaction is unknown, but it is most possible it is the same as formation of oxyazobenzene from benzolediazonium-nitrate and barium carbonate. The sodium salt of oxyazotetrazole exists as yellow crystaline plates, relatively insensitive to friction or impact, but exploding with terrible violence on heating. The barium salt of oxyazotetrazole is also known, it is a yellow compound crystallizing into a needle shape with 4 molecules of water. On prolonged boiling the salt slowly decomposes with formation of gaseous products, attempts to isolate free acid by action by action of strong acid solution on sodium salt resulted in formation of a solution, containing an unidentified substance which readily decomposed in solution. Structure of substance was not discovered because of very small amounts were available for analysis.
2. 5,5’-Diazoaminotetrazole H(N4C)-N=N-NH-(CN4)H?H2O. This substance is the product of condensation of diazotetrazole with 5-aminotetrazole. In its pure form it is almost colorless, lustrous plates with double raytrayce, and crystallizes with one molecule of water[4]. The substances contain three acidic hydrogen atoms, which can be replaced by metal ions. There are some patents, proposing salts of diazoaminotetrazole as components of electro-igniter mixtures, and as sensitizers for conventional primary explosives. Monosodium salt of diazoaminotetrazole NaC2N11H2 exists as yellow needles, readily soluble in water [2,3,4]. Copper salt Cu3(C2N11)2 – amorphous precipitate with an olive-green color, almost insoluble in water, insoluble in organic solvents. Sensitive to impact and friction, on strong grinding in mortar, only part of product is exploded (whitch was directly below the pestle), explodes from fire, flash point 195-220°С[2,3,4]. Copper-ammonium salt Cu3(C2N11)2 ? 2NH3 – dark green plates, expoding from friction, impact or heating, more sensitive then simple copper salt, flash point 220-230°С, mixture with potasium chlorate, has more initiation power then same mixture with mercury fulminate [2,3,4]. Silver salt Ag2C2HN11?H2O – white amorphous powder, exploding on heating or strong friction. Barium salt Ba3(C2N11)2?8H2O - yellow plates, explodes on moderate heating [2,3,4].
Monosodium salt synthesis[2]: 25g of aminoguanidine nitrate is dissloved in 125 ml of warm 12-13% acetic acid, 12.5g of sodium acetate is added and the mixture is cooled to 8-10°С and a solution of 17.5g sodium nitrite in 75 ml H2O is added with stirring over 30-40 minutes. Temperature of reaction mixture must be kept below 12-15°С all times, perfectly around 5-7°С. After addition of sodium nitrite, the solution is taken out from the cooling bath and is allowed to sit at room temperature, soon after that evolution of nitrogen is started, sometimes accompanied by heating up to 25°С. Aproximately after 12 hours, gas evolution stops and precipitation of product is starts, and is completed after 24 hours. Precipitated crystals are filtered off, washed with ice cold water acidified with acetic acid and is dried at room temperature.
Copper salt synthesis[2]:43g of monosodium salt is dissolved in 350 ml of water, with added 23 ml of 25% sodium hydroxide. After monosodium salt is fully dissolved, 350 ml of 10% copper sulphate solution is added slowly with stirring. Olive-green precipitate is filtered off, washed with water, ethanol, either and dried at room temperature. Copper-ammonium salt can be prepared by threatment of precipitated copper salt with strong ammonia solution, copper salt dissolves, and long green prisms of copper-ammonium salt crystals precipitate short after.
3. Bis-diazotetrazolylhydrazine (H(CN4)-N=N-NH-)2 – yellowish crystaline plates, with characteric star like edges. Poorly soluble in water and organic solvents [2,3,4,5]. Due to the high nitrogen content (14 nitrogen atoms and only 2 carbon atoms), the substance is an extremely powerful explosive. Pure product, thoroughly washed by ethanol melts at 120-123°С, where as the crude product explodes with terrible violence when heated to 90°С. Quite stable at room temperature, but very sensitive to friction and impact, violently explodes when rubbed with a glass rod or spatula. The action of alkaline solutions result in fast decomposition to 5-azidotetrazole, 5-aminotetrazole, nitrogen, ammonia and dicyan. Slowly decomposed when stored underwater due to hydrolysis. Can form extremely explosive salts, if water suspension of bis-diazotetrazolylhydrazine at 0°С is treated with a 20% solution of copper sulphate, greenish crystalls of copper salt are formed. Copper salt is extremely sensitive to friction, impact and fire, flash point 185°С. Bis-diazotetrazolylhydrazine is one of the richest on nitrogen from all known organic substances, containing 87.5% nitrogen.
Synthesis of bis-diazotetrazolylhydrazine[2]: 0.75g of hydrazine chloride and 1.5g of sodium acetate are dissolved in 3 ml of water, solution is cooled in a sodium chloride/ice bath and a solution of 1.9g diazotetrazole (Note №1) is slowly added with stirring. The yellow amorphous precipitate is washed with ice cold water, alcohol, and ether and is dried at room temperature. This work requires the greatest caution, due the extremely explosive nature of synthesized substance.
Hazard Notes:
1. Solutions of diazotetrazole are extremely explosive and can explode spontaneously, so appropriate safety measures should be taken into account all the times. It’s not recommended to make amounts bigger than those presented in the synthesis procedure due to safety reasons. All works should be carried out by experienced chemist, with the greatest caution.
References:
1. Annalen der Chemie, 273-2,3, pp 144-160, 1893, J.Thiele, J. T. Marais, “Tetrazolderivate aus Diazotetrazots?ure”.
2. P.F. Bubnov “Primary Explosives And Initiation Devices” part 1, Moscow, 1940, pp 311-314.
3. L.I. Bagal “Chemistry And Technology of Primary Explosives”, Moscow, 1975, pp 399-401.
4. L.I. Khmelnitskij “Handbook Of Explosive Materials” Part 2, Moscow, 1961, pp 89-90,96.
5. Patents DE362433C1, DE400814C1.
Some photos for this chapter
Description: Synthesis of diazoaminotetrazole from aminoguanidine, photos show amorhous grape like agglo- merates of monosodium salt of diazoaminotetrazole free floating in mother liquer, on filter and in dry state.
Description: Left photo is solution of diazoaminotetrazole trisodium salt, with characteristic red-yellow color rights photo is amorphous powder of copper diazoaminotetrazolate, obtained by mixing with copper sulphate.
5,5’-Azotetrazole and derivatives
5,5’-Azotetrazole salts were first prepared by Rathsburg by action of permanganate on salt of 5-aminotetrazole in presence of excessive ammount of alkali. Free 5,5’-azotetrazole is unstable and attempts to make it in free state by action of strong acids have failed – free 5,5’-azotetrazole imidately decomposed to tetrazylhydrazine, nitrogen and formic acid. Nitric acid causes deeper decomposition with formation of tetrazylazide. Reaction with warm sulphuric acid goes on usual way, but then cold acid is used reaction results in formation of unindentified brown explosive solid. Azotetrazole is two basic acid and forms stable salts with metallic ions. All salts of 5,5’-azotetrazole are explosive and have characteric yellow color in solution[1].
Discovery of 5,5’-azotetrazole is result of first, unsuccessfull attempts to prepare 5-nitrotetrazole. 5-Aminotetrazole contains amino group, whitch can be oxidised, what resulted in idea to make 5-nitrotetrazole by oxidation of 5-amino tetrazole by strong oxidisers, this attempts resulted in formation of yellow solution with yellow collor characteric for azo compounds, evaporation of water resulted in isolation of sodium salt of 5,5’-azotetrazole. More extensive view on reaction mechanism revealed that 5-nitrotetrazole probably forms, but in reaction conditions it imidately reacts with unreacted 5-aminotetrazole, forming 5,5’-azotetrazolates[1]:
From chemical point of view 5,5’-azotetrazole is typical azocompound, having all common properties characteric for azo compounds, tetrazolic ring provide stable aromatic rings and acidic properties. Because of strong acidic properties of tetrazole rings, salts of 5,5’-azotetrazole are stable to hydrolisis, have neutral pH and are stable to prolonged boiling. Reduction of azogroup with strong reducing agents such as tin dichloride or powdered magnessium results in reduction of azo group with formation of 5,5’-bistetrazolylhydrazine. Action of acids such as hydrochloric acid results in quantative destruction of one tetrazole ring with formation of 5-tetrazylhydrazine and release of nitrogen:
Another interesting property of azotetrazole salts is reactions with water solutions of bromine, whitch leads to decomposition of both tetrazole rings with formation of derivatifes of isocyan (unknown in free state). Action of alcohol solution of strong alkalis on this derivatives results in formation of unstable gas, with strong isonitrile odor, probably gas contains some ammount of free isocyan[1]. Up to date this is only known way to produce this interesting exotic compound. Rection scheme is shown on picture below:
5,5’-azotetrazole is important intermediate in synthesis of many other tetrazoles, many of them can be used to make compounds with even more nitrogen and higher energetic properties. Salts of azotetrazole itself are proposed for use in primary explosive mixtures and igniter compositions, for example basic lead salt was widely advertised as component of electro-igniter mixtures; copper, lead and silver salts were proposed as primary explosives; dihydrazinium salt was proposed as new high energetic, high nitrogen material, low sensitive to friction and impact and containing 85.5% of nitrogen.
Generaly salts of 5,5’-azotetrazole are crystalline solids, containing different ammount of crystallization water, colored in solution with yellow collor, characteric for azocompounds. Anhydrous salts are usualy sensitive to impact and friction and easily explode from fire, hydrated salts are less sensitive and can be grinded in mortar safely, on contact with flame they only fizz and crackle, whole mass explodes only after complete dehydration. Salts of alkali metalls are good soluble in water, salts of alkaline-earth metalls are good soluble in hot water, copper, silver, mercury and iron salts are almost insoluble [2,3,4].
Sodium salt of azotetrazole Na2C2N10 is yellow crystalline plates good soluble in water. From boiling water salt crystalizes with 5 molecules of water, salt solution has neutral pH. Then heated to 30°C solid salt losses 2 molecules of water, and at 70°C dehydrates completely. Hydrated salt is relatively low sensitive to friction and flame, can be safely grinded in mortar, on action of flame it only crackles and fizz. Anhydrous salt on friction, heating or impact explodes with loud report, explosion of 10g in Trauzl bomb gives expansion 120 ml. Flashpoint is 245-250°C. [2,3,4]
Potassium salt K2C2N10?5H2O – is good crystallizing needles or plates, very soluble in water, quckly dehydrating in air stream. Barium salt BaC2N10?5H2O – yellow needle like crystalls, badly soluble in cold water and good soluble in hot water, at 140°C losses only 3 molecules of water. Calcium salt СaC2N10?8H2O – small yellow prisms, badly soluble in cold water and good soluble in hot water, at 110C losses only part of crystallization water, further dehydrations proceeds slowly only on prolonged heating at 140°C. [2,3,4]
Iron salt FeC2N10 – black colloidal precipitate, after 3 hours of standing and passing air stream to solution, is filtered out as small dark brown crystalls. Badly soluble in water and alcohol, unsoluble in acetone, soluble in alkali solutions. Not explodes on friction and impact, flashpoint 164-165°C. Lead salt PbC2N10?5H2O – orange crystalls insoluble in water and organic solvents, soluble in alkali and acid solutions. Storage at 40C for one month not caused any changes. Puffs on action of flame, explodes on friction or impact, flashpoint 199-202°C. Zinc salt – yellow lustrous rhombic crystalls (after passing air stream to solution), slightly soluble in water, not explodes from friction or impact, flashpoint 176°C, slowly decomposes above 60°C. [2,3,4]
Copper salt CuC2N10 – sky blue crystalline precipitate (after passing the air-stream), almost insoluble in water, very sensitive to impact, stab, friction and flame. Silver and mercury salts Ag2C2N10 and HgC2N10 – unsoluble in water, in dry state explodes on sligtest stimulus (even on touch), in difference with other salts, these are unsoluble in deluted HNO3, expode more violently then corresponding azides. [2,3,4]
Synthesis of sodium 5,5’-azotetrazolate[2]: 51g of 5-aminotetrazole monohydrate is dissolved in 500 ml of 15% solution of sodium hydroxide, resulted solution is heated to 100C (Note №1) and 70g of pottasium permaganate is added slowly by portions, until coloration of solution no longer dissappear. While permanganate is added liquid can begin to boil because of strong heat evolved by redox reaction. After oxidation is completed excess of potassium permanganate is destroyed by
addition of 1-2 ml ethanol, hot solution is filtered from voluminous precipitate of manganesse dioxide and is concentrated to 1/3 of initial volume on steam bath. Product crystalizes as beautiful yellow prysms, yield is 70% (Note №2).
Notes:
1. According to some sources absence of excess alkali in reaction mixture during oxidation, can lead to formation of highly toxic and dangerous hydrogen cyanide. Alkalis transfrom unstable tetrazole rings to highly resonance-stabilized tetrazolate anions, and simplify condensation of reaction intermediates.
2. Potassium permangantate is strong oxidizer capable to oxidize water. At room temperature this reaction proceeds slowly, but at 100°C this reaction accelerates greatly and part of potassium permanganate is destroed by water, before it have time to react with aminotetrazole. Reaction equation shown above includes this side reaction.
Synthesis of other 5,5’-azotetrazolates[2]: Potassium salt of 5,5’-azotetrazole is synthesised in similar way, but sodium hyrdoxide is replaced with potassium hydroxide. Potassium salt is a lot more soluble then sodium salt, and solution must be concentrated much futher before product become to precipitate. Barium salt can be made by mixing solution of sodium salt with solution of soluble barium salt, barium salt is badly soluble in cold water and precipitate as yellow crystaline material upon cooling. Iron salt can be made by reaction of 30% solution of sodium salt with saturated solution of iron sulphate at 25°C, zinc salt is made in same manner but solution of zinc sulphate is used. Lead salt can be made by metathesis reaction of 15% solution of sodium salt with 20% solution of lead nitrate at 90-95°C, precipitate is left to settle for 3 hours and then filtered out from solution. Copper salt form when 10% solution of sodium salt is mixed with saturated copper sulphate solution, product is formed in colloidal state, but can be crystallized by passing stream of air to reaction mixture for 3 or more hours. Resulted product is filtered, washed with water, alcohol and either and is dried at 40°C. Mercury and silver salts are almost insoluble, and are easily precipitated by mixing solutions of sodium salt and soluble mercury or silver salt.
Additional Information
1. Dihydrazinium 5,5’- azotetrazolate (N2H5)(N4C-N=N-CN4)(N2H5). This substance has very large possitive heat formation and contains about 85% nitrogen[5]. Proposed for use as high preformance secondary explosive, with very large volume of explosion products. Salt containing 2 molecules of crystallization water is yellow needle-like crystalls, perfectly soluble in water and alcohol, crystallization water can be removed by heating in vacuum at 100C. Heat of formation is about 858 KJ/mol, heat of explosion is 4.378 MJ/kg, density if hydrate is about 1.564 g/cm3, calcutated detonation velocity is 6330 m/sec, calculated detonation pressure is 247 kbar. Explosion products are: N2, CH4, H2, volume of gaseous explosion products is 974 l/kg. Substance is weakly sensitive to shock (no explosion with 5 kg weight and drop height 50 cm), friction, electrostatic discharge, explodes on rapid heating. Substance is stable in storage at room temperature. May be prepared by action of hydrazine sulphate on hot solution of barium 5,5’-azotetrazolate. Precipitated barium sulphate is removed by filtration and mother liquer is concentrated on water bath until first crystalls appear, mixture is then cooled and product is collected by filtration.
2. 5,5’-Bis-tetrazolyl-hydrazine H(N4C)-NH-NH-(CN4)H. Substance is white amorphous powder with melting point at 240-241°C, explodes then heated to higher temperatures [1,6]. Heat of combustion (in closed volume) is 459.9 kcal/mol, standart heat of formation is 135 kcal/mol. Weakly soluble in boiling water, insoluble in organic solvents, soluble in concentrated hydrochloric acid, addition of ammonia or alkalis to such solution results in precipitation of corresponding salts. Alkaline solutions on exposure to light are readily oxidised salts of 5,5’-Azotetrazole, mercuric and silver salts transform to azotetrazolates on boiling in concenrated hydrochloric acid. 5,5’-Bis-tetrazolyl-hydrazine is sensitive to impact (50% explosions with 2 kg weight and drop height 32 cm), explodes when dropped to hotplate heated to 239°C. Some simple and double salts of 5,5’-bis-tetrazolyl-hydrazine can be used as primary explosives.
Barium salt Ba(C2N10H2)?H2O – pale yellow needle like crystalls, slightly soluble in water, bulk density 1.01 g/cm3, explodes then heated to 265C, heat of decomposition is 1.12 MJ/kg. In form of hydrate has low sensitivity to shock and friction. Lead salt Pb(C2N10H2) – yellow amorphous powder with bulk density 1.05 g/cm3, explodes then heated to 160°C, heat of decomposition is 1.22 MJ/kg. Sensitive to shock (50% explosions on 24g ball drop from 125 cm height) and friction (50% explosions with 4kg weight, and slide speed 8 m/sec). Mercuric salt Hg(C2N10H2) – brown amorphous powder, bulk density 0.75 g/cm3, explodes then heated to 232°C, heat of decomposition is 1.21 MJ/kg. Has low sensitivity to impact, sensitive to friction (50% explosions with 4kg weight and sliding speed of 32 m/sec) [6].
Synthesis of 5,5’-Bis-tetrazolyl-hydrazine[6]: An amount of 23 g sodium 5,5’-azotetrazole was mixed with 100 ml of distilled water and 50 g of magnesium powder and subjected to gentle reflux for about 3 hours A reaction occurred, at the end of which the yellow solution became almost colourless or sometimes pale yellow. Excess magnesium was removed by filtering the hot solution The filtrate was treated with 50 ml 50% HC1 to precipitate 5,5'-bis-terazolyl-hydrazine as a white crystalline material. The dried material weighed 15 g (89% yield) and decomposed at 518 К when heated in a glass capillary tube in an electrically heated melting point apparatus.
Salts of 5,5’-Bis-tetrazolyl-hydrazine[6]: 5,5’-Bis-tetrazolyl-hydrazine was neutralised to pH 7 with 10% NaOH solution Its concentration was adjusted to 0.1 M and the solution taken into a stainless steel beaker and heated to 60°C on a water bath A 0.1 M solution of an M1* salt (barium, lead or mercury) was placed in a dropping funnel and added dropwise over a period of 10-15 min under stirring. The contents of the beaker were further stirred for a period of 15 min at the same temperature. After cooling to room temperature, they were filtered and washed first with water and then with ethanol (95%). The resulting bis-tetrazolyl-hydrazine salts dried at room temperature for 3 to 4 hours and then in an air oven at 70°C for 1 hour. Yields varied from 70 to 90% in different experiments. In the case of the barium salt, the yield was particularly low and not consistent, this may be due to its moderate solubility in water.
3. Isocyanogen tetrazide (N3)2=C=N-N=C=(N3)2. Substance is formed by action of sodium azide on isocyanogen tetrabromide, whitch can be made by action of bromine on solution of sodium 5,5’-azotetrazolate, or better on 5,5’- bis tetrazolyl-hydrazine. Isocyanogen tetrazide is colorless needle like crystalls, melts without decomposition at 89°C, small ammounts on slow heating to 150°C can be sublimed. On shock or fast heating explodes with terrible violence, contais about 89 mass percent of nitrogen. Soluble in acetone, alcohols, either, alyphatic and aromatic hydrocarbons, chlorinated hydrocarbons, weakly soluble in water. Proposed for use as powerfull primary explosive, but is too sensitive for industrial use [7].
Synthesis of isocyanogen tetrabromide[1]: Solution of 1 mol 5,5’-bis-tetrazolyl-hydrazine is added to bromine water containing 8 mol of bromine. Mixture is heated on water bath until all dibromformaltetrazylhydrazone, witch is firs precipitated is converted to brown oil. Brown oil is separated on separation funell and is distilled with water steam, this results in formation of fast solidifying isocyanogen tetrabromide with good yield. Isocyanogen tetrabromide is recrystallized from small ammount of glacial acetic acid, by this firstly slightly yellow colored initial product containing some free bromine crystalizes as completely colorless lustrous plates, quickly loosing luster in dessicator, melting point of product is 42°C.
Synthesis of isocyanogen tetraazide[7]: 0.1 g of isocyanogen tetrabromide (purified by steam distillation and sublimation and used immediately after preparation) was dissolved in 1.0 ml. of acetone and cooled at 0°C. A solution of 0.09 g. (about 30% excess) sodium azide (activated by rubbing with a trace of N2H4*H2O and precipitated from a little water with acetone) in 0.6 ml. of water, cooled to about 0°C, was added dropwise while the reaction mixture was stirred by passing therethrough a slow stream of nitrogen. After one hour the cooling bath was removed while stirring was continued for three hours, allowing the reaction mixture to warm up to 30°С. An amount of 5.0 ml. of ice-cold water was added then and the mixture was kept for one half an hour in an ice bath. The formed crystals were filtered, washed with 5.0 ml. of ice-cold water and dried on the filter by air. Yield: 45 milligrams (76 percent). White needles, melting point: 89°С.
4. 5-Hydrazotetrazole H(N4C)-NH-NH2. This substance is formed by action of strong acids on salts of 5,5’-azotetrazole. Colorless crystals, soluble in alkali and ammonia, weakly soluble in water, insoluble in ethanol, benzole, either. Melting point is 199C, explodes then heated to higher temperatures[1,8]. Can be made by action of 2 mol of dilute hydrochloric acid on 1 mol of sodium 5,5’-azotetrazole, during this operation one tetrazole ring of azotetrazole is destroyed leaving nitrogen and formic acid. Same reaction takes place with hot diluted sulphuric acid, however then sulphuric acid is cold some brown colored explosive product is formed, it’s constitution is unknown. Care should be taken not to use nitric acid for hydrazotetrazole production, because this reaction leads to formation of very dangerous 5-azidotetrazole [8]. Some double salts of tetrazylhydrazine are proposed for use as primary explosives, for example 5-hydrazotetrazolato mercury (II) perchlorate [Hg(HN4CNHNH2)](ClO4)2 is recommended for use in laser initiated detonators, and is extremely sensitive to impulse laser beam in optical an IR areas of spectra and can be used in form of film shape charges with opticaly transpa-rent polymeres[9]. Flash point is 186C (5 sec delay), density is 3.45 g/cm3, detonation velocity is about 6 km/sec at 3.4 g/cm3 density. Minimal ininitating charge vs RDX is 0.015g. Salt is not hygroscopic, insoluble in water and majority of organic solvents, soluble in dimethylsulfoxide. Can be made by action of mercury (II) salt on solution of 5-hydrazotetrazole in 5-70% perchloric acid on heating.
Synthesis of 5-hydrazotetrazole[8]: To a solution of disodium azotetrazole pentahydrate (10.0 g) suspended in water (100 ml.) was added hydrochloric aeid (25 ml 5N). The solution was warmed on a water bath until the gas evolution finished, then was evaporated to dryness from water three times to remove the hydrochloric acid. The residue was dissolved in a minimum of hot water and a hot solution of sodium acetate (10.0 g) in water (10 ml) was added.The apparatus was flushed out with carbon dioxide and the solution allowed to cool to give 5-hydrazino-tetrazole as white prisms with a yield of 2.5g, melting point 195-198°С.
5. 5-Azidotetrazole H(N4C)-N3. Tetrazylazide is one of organic substances richest in nitrogen. It’s crystalline subsance crystallized from benzene, trichloromethane or carbon tetrachloride in form of white needles. Readily soluble in water, acetone, alcohol, slightly soluble in benzene, insoluble in ligroine, either [2,3,10,11,12,13]. On slow heating melts at 72-74C, at 217C explodes without melting. Extremely sensitive to impact and friction, on slight shock or friction explodes with terrible violence demonstrating extreme brisance effect. Stable on storage in pure state, but in presence of some impurities can explode spontaneusly. For example solution of tetrazylazide in acetone in presence of acetic acid can detonate sponta-neously on storage, but same solutions in water and alcohol are stable. Density of 5-tetrazylazide, measured from X-Ray diffraction is 1.72 g/cm3, estimated heat of formation is 611 kj/mol, estimated detonation velocity (max) is 8986 m/sec [11]. Ammonium salt NH4CN7 – can be made by blowing ammonia gas through solution ot tetrazylazide in benzene. White crystaline powder, readily soluble in water and methanol, slightly soluble in ethanol and benzene[2,4]. Less sensitive to friction and impact than free tetrazylazide, but more sensitive towards heating. Salt is stable at room temperature. Potassium salt KCN7 – white lustrous plates or needles, exploding without melting at 72-73C. Readily soluble in water, ethanol and acetone, insoluble in benzene and either. Expodes then dropped on hotplate heated above 70C, but on slow heating not explodes until 200C if not touched by spatula. Extremely sensitive to shock, friction and slightest pressure. Explodes with great violence on attemp of vacuum funell filtration or then slighlty pressed by spatula. Brisance effect is so great that even 0.01g ammount can cause dammage [4,10,13]. Sodium salt NaCN7 – lustrous white plates, explode without melting, properties are same as for potassium salt [4,10,13]. Barium salt Ba(CN7)2 – very hygroscopic crystalls, when ignited by flame deflagrates with red flame and strong sound, soluble in acetone and pyridine, insoluble in absolute alcohol, benzene, trichloromethane and petrol either. Sensitivity to shock is 50% explosions with 500g weight and 35 cm drop height [4]. Lead salt Pb(CN7)2 – crystaline substance insoluble in water and organic solvents. Explodes with red flash when touched by flame, sensitivity to impact is above 100 cm with 5 kg weight[4]. Mercury salt Hg(CN7)2 – explosive solid insoluble in water[4]. Copper salt Cu(CN7)2 – amorphous powder with green color, insoluble in water. Extremely sensitive to impact, impact and flame, flashpoint is 170C [2,4]. Silver salt AgCN7 – insoluble in water, explodes even in wet state on heating or slightest touch. Extremely brisant, unstable on storage – explodes spontaneously [4,12].
Synthesis of 5-azidotetrazole[10]: From 5-hydrazotetrazole and nitrous acid. A solution of 2.3 g. (0.023 mole) of tetrazolyl hydrazine and 2 g. (0.029 mole) of sodium nitrite in 100 ml. of water was prepared. It was cooled to 5C and 5 ml. of concentrated hydrochloric acid was added. There was a small amount of gas evolution and the solution turned a cloudy yellow. The mixture was filtered and evaporated to dryness on a steam-bath. The light yellow plates thus obtained were extracted with acetone and the yellow acetone solution on evaporation to dryness at room temperature gave a yellow solid. This solid was heated with benzene and a brown oil formed on the bottom. When the benzene layer was decanted off and cooled, long white needles were obtained. The brown oil was discarded. The yield for two runs was 20-22%. When the brown oil was extracted with more benzene, the yield was raised to 45-48%. The white needles melted sharply without decomposition at 72-73C and exploded when heated in a flame or placed on a hot bar, 217C or over. Tetrazolyl azide is also obtained as fine white needles from chloroform and carbon tetrachloride. It is very soluble in water, absolute ethanol or absolute ether.
Synthesis of potassium 5-azidotetrazolate: From diaminoguanidine and nitrous acid [10]. A solution of 3.04 g. (0.02 mole) of diaminoguanidine nitrate, 1.47 g. (0.015 mole) of potassium acetate and 1.74 g. (0.029 mole) of glacial acetic acid in 30 ml. of water was prepared and cooled to 0C (some solid reprecipitated). A solution of 3.74 g. (0.044 mole) of potassium nitrite in 10 ml. of water was added slowly keeping the temperature at 0-5C. The solution thus obtained was allowed to stand in an ice-box for two to four days. It was then evaporated nearly to dryness under vacuum (water aspirator) and in a stream of pure nitrogen. Long, white, prismatic needles of potassium nitrate were filtered off and washed with ethanol. An additional small amount of potassium nitrate came out of the filtrate on the addition of alcohol and was also filtered off. The filtrate was evaporated to dryness on a steam-bath and the yellow solid obtained was washed with absolute ether to remove the acetic acid and then extracted with acetone. The residue consisted of salts and was discarded. Absolute ether was added to the yellow acetone solution to the cloud point and allowed to stand. Fine cream colored needles
formed and were filtered, and the filtrates saved. These needles were dissolved in the minimum amount of acetone and reprecipitated by the addition of absolute ether. This time beautiful, shiny white plates of potassium tetrazolyl azide (11) were obtained. Further purification was effected in the same manner. By adding more absolute ether to the filtrates an additional amount of product was obtained. Potassium tetrazolyl azide explodes violently when heated in a flame, giving a purple color. It will also explode when placed on a hot melting-point bar (60C or above), but when heated slowly it is stable to about 200 unless touched with a spatula. It can also be detonated by light pressure.
Synthesis of sodium 5-Azidotetrazole: From Cyanogen Bromide and Sodium Azide [13].To a solution of sodium azide (3.8 g, 0.058 mol) in water (10 ml) at 0-5C was added (15 min) finely pulverized cyanogen bromide (6.8 g, 0.064 mol). The mixture was stirred at 0-5C for 30 min, and the cold solution was then extracted with ether (2 x 15 ml). The water layer was evaporated to dryness at 50C (I mm). (Caution: The product may detonate if pressure is changed rapidly when the product is dry.) The resulting salt was extracted with hot acetone (3 X 25 ml). The extract was concentrated to about 35 ml and ether was added to precipitate Sodium 5-azidotetrazolate(2.56 g, 66%).
Synthesis of ammonium-5-azidotetrazole: From Cyanogen Bromide and Sodium Azide [13]. An aqueous solution of 5-azidotetrazole is prepared as described from cyanogen bromide (6.2 g, 0.059 mol) and sodium azide (7.6 g, 0.117 mol) was acidified to pH 1 and extracted with ether (3 X 50 ml). The dried ether extract was saturated with anhydrous ammonia and filtered to separate pure ammonium-5-azidotetrazole as a white, crystalline solid (15 2 g, 95%), mp 185-186C. Hazard note: Tetrazylazide is extremely sensitive and powerfull explosive. Dry potassium salt is extremely sensitive to friction, heat, electric shock, and pressure. For example, a dry sample of potassium 5-azidotetrazole at 1-mm pressure will usually detonate if brought rapidly to atmospheric pressure. Great care and adequate protective equipment (shields, leather gloves, and jacket) should be used when preparing even small quanties of the dry compound. Samples larger than 0.1 g are best handled remotely. The salt can be prepared and handled safely in aqueous solution or as a free-flowing solid when moistened with water or mixed with an equal weight of mineral oil. We have prepared acetone solutions without event, but Lieber reports that such solutions containing traces of acetic acid may detonate and in this respect above procedure is safer. Pure dry 5-azidotetrazole is less sensitive than its potassium salt but the same handling precautions apply. Ammonium-5-azidotetrazole is still less sensitive to shock but detonates when heated rapidly to 190C. References:
1. Annalen der Chemie, 303-1, pp 57-75 , 1898 , J.Thiele “Ueber Azo- und Hydrazoverbindungen des Tetrazols”.
2. P.F. Bubnov “Primary Explosives And Initiation Devices” part 1, Moscow, 1940, pp 314-316.
3. L.I. Bagal “Chemistry And Technology of Primary Explosives”, Moscow, 1975, pp 395-40
4.
4. L.I. Khmelnitskij “Handbook Of Explosive Materials” Part 2, Moscow, 1961, 84-85(azoTz), pp 16-19(tetrazylazide).
5. Inorg. Chem. 2001, 40, pp 3570-3575 ; A. Hammerl, T. M. Klapo1tke, “A New High-Nitrogen High-Energetic Material”.
6. Journal of Hazardous Materials, 9 (1984) 291-303 ; G.Reddy, A.K. Chatterjee “A Study On Thermal And Explosive Properties Of Hydrazotetrazoles”.
7. US Patent 2990412, “Isocyanogen tetraazide and it’s preparation”, patented 27 June 1961.
8.A. J. Barratt, L. R. Bates, J. M. Jenkins, J. R. White, “Reactions of the azotetrazole anion with dilute mineral acids”, U. S. Nat. Tech. Inform. Serv. AD Report No. 752370, 1971 [C.A. 1973, 78, 124508].
9. Russian Patent 2225840C2.
10. J. Am. Chem. Soc., 73, 1313 (1951) ; E.Lieber, D.E.Evering “The Reaction of Nitrous Acid with Diaminoguanidine in Acetic Acid Media. Isolation and Structure Proof of Reaction Products”.
11. Zeitschrift für anorganische und allgemeine Chemie Volume 634 Issue 6-7, Pages 1051 – 1057 ; J?rg Stierstorfer, Thomas M. Klap?tke, Prof. Dr. , Anton Hammerl, Robert D. Chapman “5-Azido-1H-tetrazole Improved Synthesis, Crystal Structure and Sensitivity Data”.
12.A. J. Barratt, L. R. Bates, J. M. Jenkins, J. R. White, “Reactions of the azotetrazole anion with dilute mineral acids”, U. S. Nat. Tech. Inform. Serv. AD Report No. 752370, 1971 [C.A. 1973, 78, 124508].
13. J. Org. Chem., Vol. 37, No. 19, 1972 pp 2966-2969 ; F.D. Marsh “Cyanogen Azide”.
如何写先进个人事迹
如何写先进个人事迹 篇一:如何写先进事迹材料 如何写先进事迹材料 一般有两种情况:一是先进个人,如先进工作者、优秀党员、劳动模范等;一是先进集体或先进单位,如先进党支部、先进车间或科室,抗洪抢险先进集体等。无论是先进个人还是先进集体,他们的先进事迹,内容各不相同,因此要整理材料,不可能固定一个模式。一般来说,可大体从以下方面进行整理。 (1)要拟定恰当的标题。先进事迹材料的标题,有两部分内容必不可少,一是要写明先进个人姓名和先进集体的名称,使人一眼便看出是哪个人或哪个集体、哪个单位的先进事迹。二是要概括标明先进事迹的主要内容或材料的用途。例如《王鬃同志端正党风的先进事迹》、《关于评选张鬃同志为全国新长征突击手的材料》、《关于评选鬃处党支部为省直机关先进党支部的材料》等。 (2)正文。正文的开头,要写明先进个人的简要情况,包括:姓名、性别、年龄、工作单位、职务、是否党团员等。此外,还要写明有关单位准备授予他(她)什么荣誉称号,或给予哪种形式的奖励。对先进集体、先进单位,要根据其先进事迹的主要内容,寥寥数语即应写明,不须用更多的文字。 然后,要写先进人物或先进集体的主要事迹。这部分内容是全篇材料
的主体,要下功夫写好,关键是要写得既具体,又不繁琐;既概括,又不抽象;既生动形象,又很实在。总之,就是要写得很有说服力,让人一看便可得出够得上先进的结论。比如,写一位端正党风先进人物的事迹材料,就应当着重写这位同志在发扬党的优良传统和作风方面都有哪些突出的先进事迹,在同不正之风作斗争中有哪些突出的表现。又如,写一位搞改革的先进人物的事迹材料,就应当着力写这位同志是从哪些方面进行改革的,已经取得了哪些突出的成果,特别是改革前后的.经济效益或社会效益都有了哪些明显的变化。在写这些先进事迹时,无论是先进个人还是先进集体的,都应选取那些具有代表性的具体事实来说明。必要时还可运用一些数字,以增强先进事迹材料的说服力。 为了使先进事迹的内容眉目清晰、更加条理化,在文字表述上还可分成若干自然段来写,特别是对那些涉及较多方面的先进事迹材料,采取这种写法尤为必要。如果将各方面内容材料都混在一起,是不易写明的。在分段写时,最好在每段之前根据内容标出小标题,或以明确的观点加以概括,使标题或观点与内容浑然一体。 最后,是先进事迹材料的署名。一般说,整理先进个人和先进集体的材料,都是以本级组织或上级组织的名义;是代表组织意见的。因此,材料整理完后,应经有关领导同志审定,以相应一级组织正式署名上报。这类材料不宜以个人名义署名。 写作典型经验材料-般包括以下几部分: (1)标题。有多种写法,通常是把典型经验高度集中地概括出来,一
集合名词教你分清名词单复数
集合名词-教你分清名词单复数 集合名词 第一类 形式为单数,但意义可以用为单数或复数这类集合名词包括family(家庭)family,team(队),class(班),audience(听众)等, 其用法特点为:若视为整体,表示单数意义;若考虑其个体成员,表示复数意义。 比较并体会:His family is large. 他的家是个大家庭。 His family are all waiting for him. 他的一家人都在等他。 This class consists of 45 pupils. 这个班由45个学生组成。 This class are reading English now. 这个班的学生在读英语。 这个班的学生在读英语。
第二类 形式为单数,但意义永远为复数这类集合名词包括cattle(牛,牲畜)cattle,people(人),police(警察)等, 其用法特点为:只有单数形式, 但却表示复数意义,用作主语时谓语用复数;不与a(n) 连用,但可与the连用(连用)。 如:People will laugh at you. 人们会笑你的。 The police are looking for him. 警察在找他。 Many cattle were killed for this. 就因为这个原因宰了不少牲畜。 注:表示牲畜的头数,用单位词head(单复数同形)。如:five head of cattle 5头牛,fifty (head of) cattle 50头牛 第三类 形式为复数,意义也为复数这类集合名词包括goods(货物), clothes(衣服)等, 其用法特点是:只有复数形式(当然也表示复数意义,用作主语时谓
建筑名词解释
建筑名词解释,(虽然都学过,但是实际用的时候都忘了,留着以后用的着) 1、什么是相对标高?答:地面到假定水准面的铅垂距离. 什么是绝对标高? 答:地面点到大地水准面的铅垂距离. 2、什么是定位轴线?答:定位轴线是用来确定建筑物主要结构或构件的位 置及其标志尺寸的线。 3、什么是横向、纵向?什么是横向轴线、纵向轴线?答:(1)、横向, 指建筑物的宽度方向。(2)、纵向,指建筑物的长度方向。 4、什么是框架结构?答:框架结构指由柱子、纵向梁、横向梁、楼板等构 成的骨架作为承重结构,墙体是围护结构。 5、什么是建筑密度?答:建筑密度是项目总占地基地面积与总用地面积的 比值。一般用百分数表示。 6、什么是过梁?其作用是什么?答:过梁是门窗洞口上方的横梁,其作用 是承受门窗洞口上部的荷载,并把它传到门窗两侧的墙上,以免门窗框被压坏或变形。过梁的长度一般为门窗洞口的跨度加500mm。 7、什么是绿地率(绿化率)?答:绿地率是项目绿地总面积与总用地面积 的比值。一般用百分数表示。 8、什么是日照间距?答:日照间距,就是前后两栋建筑之间,根据日照 时间要求所确定的距离。日照间距的计算,一般以冬至这一天正午正南方向房屋底层窗台以上墙面,能被太阳照到的高度为依据。 9、建筑物与构筑物有何区别?答:凡供人们在其中生产、生活或其他活动 的房屋或场所都叫做建筑物,如公寓、厂房、学校等;而人们不在其中生产或生活的建筑,则叫做构筑物,如烟囱、水塔、桥梁等。 10、什么是建筑“三大材”?答:建筑“三大材”指的是钢材、水泥、木材。 11、建筑安装工程费由哪三部分组成?答:建筑安装工程费由人工费、材 料费、机械费三部分组成。 12、什么是标志尺寸、构造尺寸、实际尺寸?答:(1)、标志尺寸是用 以标注建筑物定位轴线之间(开间、进深)的距离大小,以及建筑制品、建筑构配件、有关设备位置的界限之间的尺寸。标志尺寸应符合模数制的规定。(2)、构造尺寸是建筑制品、建筑构配件的设计尺寸。构造尺寸小于或大于标志尺寸。一般情况下,构造尺寸加上预留的缝隙尺寸或减去必要的
最新小学生个人读书事迹简介怎么写800字
小学生个人读书事迹简介怎么写800字 书,是人类进步的阶梯,苏联作家高尔基的一句话道出了书的重要。书可谓是众多名人的“宠儿”。历来,名人说出关于书的名言数不胜数。今天小编在这给大家整理了小学生个人读书事迹,接下来随着小编一起来看看吧! 小学生个人读书事迹1 “万般皆下品,惟有读书高”、“书中自有颜如玉,书中自有黄金屋”,古往今来,读书的好处为人们所重视,有人“学而优则仕”,有人“满腹经纶”走上“传道授业解惑也”的道路……但是,从长远的角度看,笔者认为读书的好处在于增加了我们做事的成功率,改善了生活的质量。 三国时期的大将吕蒙,行伍出身,不重视文化的学习,行文时,常常要他人捉刀。经过主君孙权的劝导,吕蒙懂得了读书的重要性,从此手不释卷,成为了一代儒将,连东吴的智囊鲁肃都对他“刮目相待”。后来的事实证明,荆州之战的胜利,擒获“武圣”关羽,离不开吕蒙的“运筹帷幄,决胜千里”,而他的韬略离不开平时的读书。由此可见,一个人行事的成功率高低,与他的对读书,对知识的重视程度是密切相关的。 的物理学家牛顿曾近说过,“如果我比别人看得更远,那是因为我站在巨人的肩上”,鲜花和掌声面前,一代伟人没有迷失方向,自始至终对读书保持着热枕。牛顿的话语告诉我们,渊博的知识能让我们站在更高、更理性的角度来看问题,从而少犯错误,少走弯路。
读书的好处是显而易见的,但是,在社会发展日新月异的今天,依然不乏对读书,对知识缺乏认知的人,《今日说法》中我们反复看到农民工没有和用人单位签订劳动合同,最终讨薪无果;屠户不知道往牛肉里掺“巴西疯牛肉”是犯法的;某父母坚持“棍棒底下出孝子”,结果伤害了孩子的身心,也将自己送进了班房……对书本,对知识的零解读让他们付出了惨痛的代价,当他们奔波在讨薪的路上,当他们面对高墙电网时,幸福,从何谈起?高质量的生活,从何谈起? 读书,让我们体会到“锄禾日当午,汗滴禾下土”的艰辛;读书,让我们感知到“四海无闲田,农夫犹饿死”的无奈;读书,让我们感悟到“为报倾城随太守,西北望射天狼”的豪情壮志。 读书的好处在于提高了生活的质量,它填补了我们人生中的空白,让我们不至于在大好的年华里无所事事,从书本中,我们学会提炼出有用的信息,汲取成长所需的营养。所以,我们要认真读书,充分认识到读书对改善生活的重要意义,只有这样,才是一种负责任的生活态度。 小学生个人读书事迹2 所谓读一本好书就是交一个良师益友,但我认为读一本好书就是一次大冒险,大探究。一次体会书的过程,真的很有意思,咯咯的笑声,总是从书香里散发;沉思的目光也总是从书本里透露。是书给了我启示,是书填补了我无聊的夜空,也是书带我遨游整个古今中外。所以人活着就不能没有书,只要爱书你就是一个爱生活的人,只要爱书你就是一个大写的人,只要爱书你就是一个懂得珍惜与否的人。可真所谓
广告培训广告术语
above-the-line advertising 线上广告 广告代理商能从媒介获得佣金(代理费)的广告,如报刊广告、广播广告、电视广告、影院广告、户外广告等。 account executive (AE) 客户经理 广告公司的业务人员职称。客户经理往往须负责下列工作:1,与客户及内部其他部门共同计划广告(planning),向各部门传达客户的诉求;2,内部协调(coordination);3,将广告设计稿提供给客户;4,监督执行政府的有关广告规章和法规(regulatory matters);5,利润管理(agency profit management)。客户经理通过计划和协调公司的服务部门,为客户提供更好的服务。 account service 客户服务 客户服务是广告代理商的中心工作,肩负着使客户满意从而建立起长期的合作关系,及推动广告代理商内部工作有效运转的任务。它是广告代理商直接同客户进行沟通、交流的一种功能。 advertising agency 广告代理商 习惯上称为“广告公司”,即《中华人民共和国广告法》中所称的广告经营者,一般设 有许多职能和业务部门。 advertising campaign 广告活动 有时称为“运动”或“战役”。广告活动包括以下四个重点:制作适当的销售信息、及 时传达给受众、选择适当的时机,用合理的成本。广告主制定一项能测定的目标后,为 达到这一目标制定广告战略,然后在市场上执行,包括:广告计划、广告制作、销售及 营销等。 advertising department 广告部 分为企业的广告部和媒介的广告部。企业的广告经理负责拟定、审核及实施企业的广告 计划。一般也是负责有关广告的具体工作。媒介的广告部经理负责出售报刊等的版面, 广播、电视的时间等。 airport advertising 机场广告 利用机场的候机室及在机场内其他各种场地和设备上制作刊出的广告,也包括在指示牌 上制作的广告。 Appeal 诉求 广告通过媒介向目标受众诉说,以求达到所期望的反应。诉求是制定某种道德、动机、 认同,或是说服受众应该去做某件事的理由。诉求分三类:理性的、感性的和道义的。 诉求所用语句应具有强烈的感染力。 area sampling 区域抽样 群体抽样的一种形式。样本空间按区域进行划分,选定某抽样区域,如一个县、一个行 政区、一个街区,从中确定调查对象。 Audience 受众 接受广告的公众,也就是广告的对象。通过任何广告媒介接触的观众或听众,都有数量 、特征方面的不同需要考虑到。这些不同可使广告做到有的放矢。
英语中的集合名词
英语中的集合名词是经常考查的一个考点,它主要涉及集合名词的可数性、单复数意义、主谓一致、恰当的修饰语等。为了便于理解和记忆,我们将一些常考的集合名词分为以下几类,并分别简述其有关用法特点: 这类集合名词包括family (家庭),team (队),class (班),group (组),audience (听众)等,其用法特点为:若视为整体,表示单数意义;若考虑其个体成员,表示复数意义。 His family is large. 他的家是个大家庭。 His family are all waiting for him. 他的一家人都在等他。 This class consists of 45 pupils. 这个班由45个学生组成。 This class are reading English now. 这个班的学生在读英语。 The football team is playing well. 那个足球队打得非常漂亮。 The football team areshavingsbath and are then coming back here for tea. 足球队员们正在洗澡,然后来这里吃茶点。 The family is a very happy one.那个家庭是一个非常幸福的家庭。 That family are very pleased about the news of William's success. 这类集合名词包括cattle(牛,牲畜),people(人),police(警察)等,其用法特点为:只有单数形式, 但却表示复数意义,用作主语时谓语用复数;不与a(n) 连用,但可与the连用(表示总括意义和特指)。如: People will laugh at you. 人们会笑你的。 The police are looking for him. 警察在找他。 Many cattle were killed for this. 就因为这个原因宰了不少牲畜。 注:表示牲畜的头数,用单位词head(单复数同形)。如:
建筑名词解释
『台基』建筑名词解释 台基 就是建筑物的底座。四面以砖石砌成,内多填土,地 面铺以砖石。 须弥座xūmízu?原为佛象底座。由印度传入中国後,被用作尊贵建筑的底座。它的组成为圭角、下枋、下枭、束腰、上枭、上枋等。 如故宫三大殿的台基就是简约式的须弥座。 踏跺 tàdu? 就是用来登上台基的阶级。 龙尾道 在坡道较长的情况下,会用平、坡相间形式建造的阶 榜。 慢道较为平缓的阶梯或斜道。 礓碴 jiāngchá是将砖石打侧,以其梭角所砌成的斜道,给人和马车登上台基。形状就像洗衣板。 辇道 niǎndào 有斜度的路,方便马车通行。於唐宋时期,设置於踏跺之间。後来,辇道被加上水、云、龙等雕刻,渐渐演变成今天的「御路」。 陛石bìshí用作铺设御路的石块。 如故宫保和殿北面御路的大石雕。 角石 用于宋式台基的角位,常雕刻有龙、凤、狮子等装饰。 清代台基是不用角石设计。 螭首螭是传说中有角的龙。突出的螭首雕刻设置在台基外
chīshǒu部。除美观外,它用作为排走雨水的出水口。 如天坛祈年殿的龙、凤、云三种形式的螭首。『柱』建筑名词解释 柱zhù就是建筑物的底座。四面以砖石砌成,内多填土,地面则铺以砖石。 柱础是垫著木柱的石墩。 鼓镜是指柱础凸出于地面的部份。 覆盆唐宋时期,常用的鼓镜设计,像倒转的碗。 鼓蹬 因南方天气潮湿,所以柱础都设计成较高身的鼓状石 墩,称之为鼓蹬。 檐柱yánzhù建筑物最外的一列柱子,用来支撑屋檐的重量。通常建筑物有前後两列檐柱。 中柱 位处建筑物中轴线上的柱子,用作支撑屋脊的。但中 柱不包括在山墙之内的柱子。 山柱设在山墙内,顶著屋脊的柱子。 角柱位处山墙两端,建筑物转角的柱子。 金柱除檐柱、中柱、山柱外,其他柱子都称作金柱。 外金柱距离檐柱较近的柱子为外金柱。 里金柱 「里」和「外」是相对的,距离檐柱较远的柱子便称 作里金柱。
广告公司专业术语84419
以下属于涉及面很广, 有广告领域的, 有数字设计领域的, 更有IT领域的, 因为本身数字互动营销就是个很多元很不清不楚的东东~ 我承认这很无聊, 不过事实的确是这样的, 很多概念我们圈内人士自己都搞不清楚! 那该如何沟通, 如何清楚的向客户去表述呢? 比如数字营销, 互动营销, 网络营销, 这三个概念, 能一下说明白的大概也该去中科院了. 又比如线上, 线下这个“线”能定义清楚的…废话不多(不断更新中…)——————————————————————— Advertorial (软文) 广告的一种, 即付费文章, 故意设计成像一篇普通的文章。 Appeals (述求) Emotional Appeals/感性诉求, Rational Appeals/理性诉求 Art Base(美术/设计出身) 刚入行不久的同学也许会经常被人问到:“你是Art Base 还是Copy Base?” 一开始都是被问的一头雾水! 在广告圈中创意人分出身有两个派系——Copy Base和Art Base. Copy Base指出身于文案, 文字达人(Copywriter); Art Base就是指出身于美术, 设计, 视觉达人! 比如你是某公司CD, 你在向别人介绍时可以这么用:“我是xxx公司CD, 我是Art Base!”ATL (线上) 还有BTL呢! 这个讲起来真是有的讲, 相信平时大家都会被线上线下的概念混淆的团团转…有时间要写一篇关于这两个概念的日记. 互相探讨下! 其实在无数传统广告的书籍上都有讲这两个概念的. 但是这条线究竟是啥呢? ATL即Above The Line; BTL即Below The Line. ATL可以泛指为广告; BTL则是其他销货
建设工程名词解释
建设工程名词解释 饶志辉 1 结构转换层:建筑物某层的上部与下部因平面使用功能不同,该楼层上部与下部采用不同结构类型,并通过该楼层进行结构转换,则该楼层称为结构转换层。 2 冷剂压套筒接头: 3 沥青橡胶应力吸收薄膜: 4 冷桥现象:房屋外墙转角、内外墙交角、楼屋面与外墙搭接角的区域范围,在室内温度高于室外温度时候,产生水雾吸附于墙面的现象称为"冷桥"现象,大多出现在冬季。 5 银粉、金粉:含金属涂料的油漆。 6 (越岭线)垭口:山口。 7 路灯、草坪灯、庭园灯、地灯:路灯:装在道路上照明用的灯。草坪灯:草坪灯的设计主要以外型和柔和的灯光为城市绿地景观增添安全与美丽,并且普遍具有安装方便、装饰性强等特点,可用于公园、花园别墅、广场绿化、等场所的绿化带的装饰性照明。地灯:安装在地面上或接近地面位置上的灯。 8 两带:灞河滨水生活蓝带和浐河城市生态绿带。 9 绿肺:比喻能吸收二氧化碳并释放出氧气的绿地、森林等。 10 电气元器件气候防护:潮湿、盐雾、霉菌以及气压、污染气体对电子设备影响很大,其中潮湿的影响是最主要的。通常采用浸渍、灌封、密封等措施。
11 牛腿、肩梁: 12 声发射定位信号: 13 错层结构:是指在建筑中同层楼板不在同一高度,并且高差大于梁高(或大于500毫米)的结构类型。 14 红外轴温探测系统: 15 虚拟仓库:如果建筑材料市场离得不远的话,可通过信息系统对部分材料建立一个虚拟仓库,从而实现零库存。 16 高压射流技术、真空泵技术、以及射流泵的出现、液压技术的进步、超声波检测技术: 17 WSS无收缩深孔注浆技术: 18 三轴搅拌桩:是长螺旋桩机的一种,同时有三个螺旋钻孔,施工时三条螺旋钻孔同时向下施工,是软基处理的一种有效形式,利用搅拌桩机将水泥喷入土体并充分搅拌,使水泥与土发生一系列物理化学反应,使软土硬结而提高地基强度。 19 工程地质与基坑开挖深度及地连墙墙趾关系图: 20 透水砖:也叫渗水砖、荷兰砖。 21 水幕降尘器:是一种比较传统且常用的降尘方法。 22 水泡泥技术:一种隧道爆破时降低粉尘的技术。水泡泥就是用装水的塑料袋填于炮眼内来代替一部分泡泥,装完药后将其填于炮眼内,尽量不要搞破,然后用黄泥封堵。实践表明,此法降尘效率非常高。 23 单向阀:单向阀是流体只能沿进水口流动,出水口介质却无法回流,俗称单向阀。单向阀又称止回阀或逆止阀。用于液压系统中防止
个人先进事迹简介
个人先进事迹简介 01 在思想政治方面,xxxx同学积极向上,热爱祖国、热爱中国共产党,拥护中国共产党的领导.利用课余时间和党课机会认真学习政治理论,积极向党组织靠拢. 在学习上,xxxx同学认为只有把学习成绩确实提高才能为将来的实践打下扎实的基础,成为社会有用人才.学习努力、成绩优良. 在生活中,善于与人沟通,乐观向上,乐于助人.有健全的人格意识和良好的心理素质和从容、坦诚、乐观、快乐的生活态度,乐于帮助身边的同学,受到师生的好评. 02 xxx同学认真学习政治理论,积极上进,在校期间获得原院级三好生,和校级三好生,优秀团员称号,并获得三等奖学金. 在学习上遇到不理解的地方也常常向老师请教,还勇于向老师提出质疑.在完成自己学业的同时,能主动帮助其他同学解决学习上的难题,和其他同学共同探讨,共同进步. 在社会实践方面,xxxx同学参与了中国儿童文学精品“悦”读书系,插画绘制工作,xxxx同学在班中担任宣传委员,工作积极主动,认真负责,有较强的组织能力.能够在老师、班主任的指导下独立完成学院、班级布置的各项工作. 03 xxx同学在政治思想方面积极进取,严格要求自己.在学习方面刻苦努力,不断钻研,学习成绩优异,连续两年荣获国家励志奖学金;作
为一名学生干部,她总是充满激情的迎接并完成各项工作,荣获优秀团干部称号.在社会实践和志愿者活动中起到模范带头作用. 04 xxxx同学在思想方面,积极要求进步,为人诚实,尊敬师长.严格 要求自己.在大一期间就积极参加了党课初、高级班的学习,拥护中国共产党的领导,并积极向党组织靠拢. 在工作上,作为班中的学习委员,对待工作兢兢业业、尽职尽责 的完成班集体的各项工作任务.并在班级和系里能够起骨干带头作用.热心为同学服务,工作责任心强. 在学习上,学习目的明确、态度端正、刻苦努力,连续两学年在 班级的综合测评排名中获得第1.并荣获院级二等奖学金、三好生、优秀班干部、优秀团员等奖项. 在社会实践方面,积极参加学校和班级组织的各项政治活动,并 在志愿者活动中起到模范带头作用.积极锻炼身体.能够处理好学习与工作的关系,乐于助人,团结班中每一位同学,谦虚好学,受到师生的好评. 05 在思想方面,xxxx同学积极向上,热爱祖国、热爱中国共产党,拥护中国共产党的领导.作为一名共产党员时刻起到积极的带头作用,利用课余时间和党课机会认真学习政治理论. 在工作上,作为班中的团支部书记,xxxx同学积极策划组织各类 团活动,具有良好的组织能力. 在学习上,xxxx同学学习努力、成绩优良、并热心帮助在学习上有困难的同学,连续两年获得二等奖学金. 在生活中,善于与人沟通,乐观向上,乐于助人.有健全的人格意 识和良好的心理素质.
广告专业术语
广告专业英语 英翻中 A AAAA America Association of Advertising advocacy advertising 倡导型广告Account Executive 客户主管 Account planning 客户策划 Account Services 客户部 Advertising Department 企业广告部agency 广告代理商 Art Director 美术指导 Assistant Partner 董事助理 A priori 先验估汁(演绎法) A posteriori 实测值(归纳法) analysis 分析archetype 标准受众,原型availability 空余时段 audience duplication 重复受众 AA(Account Assistant)客户助理Action Plan 行动方案 AD(Account Director)客户总监 Add Value 附加价值 Advertorial付费软文 AM(Account Manager)客户经理Analysis Tools分析工具Announcement 公告 Annual Report年报 AP (Asia-Pacific) 亚太区 AR List 任务清单 ATL (Above the Line) 线上活动Attachment附件 Audience Awareness公众认知度Awareness 认知 ad planning 广告策划 appeal 诉求B bid [bid] 广告竞标 business-to-business(B2B)advertising brand image 品牌印象 Brainstorming 头脑风暴法 Billboard 广告路牌 Blow in card 报刊广告插页 Bulldog edition 报纸的早发版 best face forward 展现最佳面貌 brand manager 品牌经理 buried position 被淹没的广告位Background material 背景材料Benchmark测试基准 BI (Behavior Identity)企业行为识别系统Bidding 竞标 Bio个人简历(Viva Resume)Boilerplate公司简介(附在新闻稿后面的关于该公司的简短介绍) Brand Loyalty品牌忠诚度 Brand Management品牌管理 Brand Planning/Designing品牌策划/设计Brand Positioning Survey品牌定位调查Brand Promotion品牌推广 Branding Strategy品牌战略 Briefing Kit资料包 Briefing情况介绍 BTL (Below the Line) 线下活动 Bundle 附赠品 Business Model商业计划 Business Philosophy 经营哲学 Business Strategy 经营战略 brand development index 品牌成长指数back- to-back 广告连播 C capitalism 资本本主义,资本运营commercial advertising 商业广告communication process 传播过程comparative advertising 比较广告consumer advertising 消费品广告consumerism 消费主义 Chief Executive Officer(CEO) 首席执行长官
初中英语名词—集合名词
初中英语名词—集合名词 这类集合名词包括family (家庭),team (队),class (班),audience (听众), party,personnel,profession,population,staff,school,team,tribe(部落,部民),union,university等,其用法特点为:若视为整体,表示单数意义;若考虑其个体成员,表示复数意义。比较并体会:His family is large、他的家是个大家庭。His family are all waiting for him、他的一家人都在等他。This class consists of45 pupils、这个班由45个学生组成。This class are reading English now、这个班的学生在读英语。The staff is /are hardworking、The audience were moved to tears、第二类形式为单数,但意义永远为复数这类集合名词包括 cattle(牛,牲畜),people(人),police(警察), clergy,faculty(教职工), herd,mankind,military,militia(民团、民兵),poultry(家禽),swine (猪),vermin,womankind等,其用法特点为:只有单数形式, 但却表示复数意义,用作主语时谓语用复数;不与 a(n) 连用,但可与the连用(表示总括意义和特指)。如:People will laugh at you、人们会笑你的。The police are looking for him、警察在找他。Many cattle were killed for this、
建筑名词解释
解构主义,巴洛克建筑,拓扑学,波普艺术,逻各斯……】建筑师应该了解的名词,你知道多少?2011年05月31 日13:30:51 (图片如果看不到就右键在新标签页中打开,疏忽了抱歉 现代主义建筑:现代主义建筑是指二十世纪中叶,在西方建筑界居主导地位的一种建筑思想。这种建筑的代表人物主张:建筑师要摆脱传统建筑形式的束缚,大胆创造适应于工业化社会的条件、要求的崭新建筑。因此具有鲜明的理性主义和激进主义的色彩,又称为现代派建筑。 巴洛克建筑:巴洛克建筑是17~18世纪在意大利文艺复兴建筑基础上发展起来的一种建筑和装饰风格。其特点是外形自由,追求动态,喜好富丽的装饰和雕刻、强烈的色彩,常用穿插的曲面和椭圆形空间。巴洛克(baroque)此字源于西班牙语及葡萄牙语的“变形的珍珠”(—barroco)。作为形容词,此字有“俗丽凌乱”之意。欧洲人最初用这个词指“缺乏古典主义均衡特性的作品”,它原是18世纪崇尚古典艺术的人们,对17世纪不同于文艺复兴风格的一个带贬抑的称呼,现今这个词已失去了原有的贬抑,仅指17世纪风行于欧洲的一种艺术风格。 解构主义:解构主义60年代缘起于法国,雅克·德里达——解构主义领袖——不满于西方几千年来贯穿至今的哲学思想,对那种传统的不容置疑的哲学信念发起挑战,对自柏拉图以来的西方形而上学传统大加责难。 解构主义建筑:解构主义建筑是在80年代晚期开始的后现代建筑的发展。它的特别之处为破碎的想法,非线性设计的过程, 有兴趣在结构的表面或和明显非欧几里得几何上花点功夫,形成在建筑学设计原则的变形与移位,譬如一些结构与大厦封套.大厦的完成视觉外观利用不可预料和受控纷乱描绘的刺激出现了无数的解构主义的"样式"。一些解构主义的建筑师受到法国哲学家德里达(Jacques Derrida)的文字和他解构的想法的影响。虽然这个影响的程度仍然对受到怀疑; 而其他人则被重申的俄国人构成主义运动中的几何学不平衡想法所影响。在解构主义,也有对其它二十世纪运动作另外的参考: 现代主义/后现代主义互相作用,表现主义, 立体派, 简约主义及当代艺术。解构主义的全面尝试,就是让建筑学远离那些实习者所看见的现代主义的束紧规范,譬如“形式跟随功能”,“形式的纯度”,“材料的真我”和“结构的表达”。在解构主义运动的历史上的重要事件包括了1982年拉维列特公园(Parc de la Villette) 的建筑设计竞争(特别德里达和彼得·艾森曼的作品并且柏纳德·楚米的得奖作品), 1988年现代艺术博物馆在纽约的解构主义建筑展览, 由菲利普·约翰逊和马克·威格利组织, 还有1989年初位于俄亥俄州哥伦布市由彼得·艾森曼设计的卫克斯那艺术中心(Wexner Center for the Arts)。 拓扑学 拓扑学,是近代发展起来的一个研究连续性现象的数学分支。中文名称起源于希腊语Τοπολογ的音译。Topology原意为地貌,于19世纪中期由科学家引入,当时主要研究的是出于数学分析的需要而产生的一些几何问题。发展至今,主要研究拓扑空间在拓扑变换下的不变性质和不变量。拓扑学是数学中一个重要的、基础的分支。起初它是几何学的一
广告业常用术语名词解释大全
广告业常用术语名词解释 创意总监 (简称CD) 主要任务是创意管理,即控制公司的创意品质. 艺术指导 (简称AD) 通常与文案人员结成创意小组,共同发想创意概念,并具体负责创意视觉化工作. 文案人员 (简称CW) 与艺术指导共同发想创意概念,并具体负责文案写作工作. 业务员 (简称AE) 汇集客户市场,产品的基本资料,得出对市场,产品,消费者的分析. AAD〔Associated Account Director〕——副客户总监 AAD〔Associated Art Director〕——副美术指导 ACD〔Associated Creative Director〕——副创作总监 AD 〔Account Director〕—客户服务总监、业务指导 AD〔Art Director〕——美术指导(在创作部可以独挡一面执行美术指导工作的美术监督) AE〔Account Executive〕——客户执行、客户服务、客户主任;预算执行者,负责广告代理商和广告主之间的一切有关业务,观念,预算,广告表现之联系 AM 〔Account Manager〕——客户经理 AP〔Account Planner〕——客户企划(分策略企划和业务企划)两种〔Artist〕——正稿员 ASM〔Area Sale Manager〕——大区销售经理 CD〔Creative Director〕——创作总监、创意总监、创意指导(CD的前身,不是撰稿人便是美术设计,因为积累了丰富的经验,并有优异的创作成绩而成为督导) 〔Copy Director〕——文案指导 CGH〔Creative Group Head 〕——创意组长 〔Computer Visualizer〕——计算机绘图员 CW〔Copywriter〕——撰稿人 DCS〔Director of Client Service〕——客户主管 ECD〔Executive Creative Director〕——执行创意总监 FA〔Finish Artist〕——完稿、画师 〔Finish Artist Group Head〕——完稿组长 GAD〔Group Account Director〕——客户群总监 GCD〔Group Creative Director〕——创意群总监
高中英语集合名词的分类梳理
高中英语集合名词的分类梳理 英语中的集合名词是高考经常考查的一个考点,它主要涉及集合名词的可数性、单复数意义、主谓一致、恰当的修饰语等。为了便于理解和记忆,我们将一些常考的集合名词分为以下几类,并分别简述其有关用法特点: 第一类:形式为单数,但意义可以用为单数或复数 这类集合名词包括family(家庭),team(队),class(班),audience(听众)等,其用法特点为:若视为整体,表示单数意义;若考虑其个体成员,表示复数意义。比较并体会: His family is large. 他的家是个大家庭。 His family are all waiting for him. 他的一家人都在等他。 This class consists of 45 pupils. 这个班由45个学生组成。 This class are reading English now. 这个班的学生在读英语。 第二类:形式为单数,但意义永远为复数 这类集合名词包括cattle(牛,牲畜),people(人),police(警察)等,其用法特点为:只有单数形式, 但却表示复数意义,用作主语时谓语用复数;不与a(n) 连用,但可与the连用(表示总括意义和特指)。如: People will laugh at you. 人们会笑你的。 The police are looking for him. 警察在找他。 Many cattle were killed for this. 就因为这个原因宰了不少牲畜。 注:表示牲畜的头数,用单位词head(单复数同形)。如: five head of cattle 5头牛,fifty (head of ) cattle 50头牛
新建名词解释
1.国内生产总值:一国在一年内所生产的物品和劳务的市场价值的总和。 2.总需求:是指在其他条件的不变的情况下,在某一给定的价格水平上人们所愿意购买的产出总量。67789787678908907798907877897889078907890;】【】】 3.总供给:在其他条件不变的情况下,所有企业愿意提供的产出总量与整体价格水平的关系。 4.实际GDP:按固定价格或不变价格统计的GDP。 5.名义GDP:用实际市场价格统计的GDP。 6.潜在GDP:指一国经济所能持续地生产的最大的产出水平。 7.边际消费倾向:当可支配收入每增加1美元,人们所增加的消费量。 8.边际储蓄倾向:指每增加的1美元可支配收入种被用来增加储蓄的部分。 9.乘数效应:是指外生指出变化1美元对总产出的影响。 10.投资乘数:指投资增加1美元会使产出以若干倍增加。 11.商业周期:是国民总产出、总收入、总就业量的波动,持续时间通常为2—10年,它以大多数经济部门的扩张或收缩为标志。 12.狭义货币供给量(M1):指在银行体系以外流动的硬币和纸币之和,再加上支票帐户存款。 13.广义货币供给量(M2):即M1加上储蓄存款、小额定期
存款和货币市场共同基金等准货币。 14.货币的交易需求:为购买商品和劳务需要进行支付的货币量。 15.货币的资产需求:在资产增值和保持流动性之间选择而持有的货币量。 16.投资的风险:意味着投资收益的可变性。 17.名义利率:衡量的是每投资1美元所获得的货币收获。 18.实际利率:衡量的是今天我们所放弃的物品在明天能为我们带来的物品的数量。 19.通货膨胀:意味着总体价格水平的上升。 20.通货紧缩:意味着总体价格水平的下降。 21.成本推进型通货膨胀:在失业率高且资源利用不足时,由成本上升所造成的通货膨胀。 22.需求拉动型通货膨胀:总需求的增长速度超过经济潜在的生产能力,使物价上升以平衡总供给与总需求的通货膨胀。 23.中央银行的贴现率:商业银行向十二个地区性联邦储备银行借款时的利息率。 24.法定准备金率:指银行必须持有一个比例的支票存款作为准备金,这个比例被称为法定准备金率。 25.非加速通货膨胀失业率(自然失业率):是指与稳定的通货膨胀率相一致的失业率。 26.奥肯法则:相对与潜在GDP,GDP每下降2个百分点,
优秀党务工作者事迹简介范文
优秀党务工作者事迹简介范文 优秀党务工作者事迹简介范文 ***,男,198*年**月出生,200*年加入党组织,现为***支部书记。从事党务工作以来,兢兢业业、恪尽职守、辛勤工作,出色地完成了各项任务,在思想上、政治上同党中央保持高度一致,在业务上不断进取,团结同事,在工作岗位上取得了一定成绩。 一、严于律己,勤于学习 作为一名党务工作者,平时十分注重知识的更新,不断加强党的理论知识的学习,坚持把学习摆在重要位置,学习领会和及时掌握党和国家的路线、方针、政策,特别是党的十九大精神,注重政治理论水平的提高,具有坚定的理论信念;坚持党的基本路线,坚决执行党的各项方针政策,自觉履行党员义务,正确行使党员权利。平时注重加强业务和管理知识的学习,并运用到工作中去,不断提升自身工作能力,具有开拓创新精神,在思想上、政治上和行动上时刻同党中央保持高度一致。 二、求真务实,开拓进取 在工作中任劳任怨,踏实肯干,坚持原则,认真做好学院的党务工作,按照党章的要求,严格发展党员的每一个步骤,认真细致的对待每一份材料。配合党总支书记做好学院的党建工作,完善党总支建设方面的文件、材料和工作制度、管理制度等。
三、生活朴素,乐于助人 平时重视与同事间的关系,主动与同事打成一片,善于发现他人的难处,及时妥善地给予帮助。在其它同志遇到困难时,积极主动伸出援助之手,尽自己最大努力帮助有需要的人。养成了批评与自我批评的优良作风,时常反省自己的工作,学习和生活。不但能够真诚的指出同事的缺点,也能够正确的对待他人的批评和意见。面对误解,总是一笑而过,不会因为误解和批评而耿耿于怀,而是诚恳的接受,从而不断的提高自己。在生活上勤俭节朴,不铺张浪费。 身为一名老党员,我感到责任重大,应该做出表率,挤出更多的时间来投入到**党总支的工作中,不找借口,不讲条件,不畏困难,将总支建设摆在更重要的位置,解开工作中的思想疙瘩,为攻坚克难铺平道路,以支部为纽带,像战友一样团结,像家庭一样维系,像亲人一样关怀,践行入党誓言。把握机遇,迎接挑战,不负初心。
英语集合名词
?集合名词: 是语言学上的一个专有名词,意指一种可用来指称一群对象的词,而这些对象,可以是人、动物、或是一群概念等事物。 例如:family (家庭),cattle (牛, 牲畜),goods (货物),baggage/luggage (行李),hair (头发, 毛发),fruit (水果) ?集合名词分类及用法特点: 第一类 形式为单数,但意义可以用为单数或复数这类集合名词 包括family(家庭),team(队),class(班),audience(听众)等。 其用法特点为: 若视为整体,表示单数意义;若考虑其个体成员,表示复数意义。 比较并体会: His family is large. 他的家是个大家庭。 His family are all waiting for him. 他的一家人都在等他。 This class consists of 45 pupils. 这个班由45个学生组成。 This class are reading English now. 这个班的学生在读英语。 第二类 形式为单数,但意义永远为复数这类集合名词 包括cattle(牛,牲畜),people(人),police(警察)等。 其用法特点为: 只有单数形式, 但却表示复数意义,用作主语时谓语用复数;不与a(n) 连用,但可与the连用(连用)。
如:People will laugh at you. 人们会笑你的。 The police are looking for him. 警察在找他。 Many cattle were killed for this. 就因为这个原因宰了不少牲畜。 注:表示牲畜的头数,用单位词head(单复数同形)。 如:five head of cattle 5头牛,fifty (head of ) cattle 50头牛 第三类 形式为复数,意义也为复数这类集合名词包括goods(货物), clothes(衣服)等。 其用法特点是: 只有复数形式(当然也表示复数意义,用作主语时谓语也用复数),但通常不与数词连用。 如:Clothes dry slowly in the rainy season. 衣服在雨季不易干。 Such clothes are very expensive. 那样的衣服很贵。 If goods are not well made you should complain to the manufacturer. 如果货物质量不好,则理应向制造商提出控诉。 第四类 形式为单数,意义也为单数这类集合名词 包括baggage / luggage(行李), clothing(衣服), furniture(家具), machinery(机器), poetry(诗), scenery(风景),jewelry(珠宝), equipment(设备)等。 其用法特点为: 是不可数名词,只用单数形式,不用不定冠词(当然更不能用数词),没有复数形式。 如:Our clothing protects us from [against] the cold. 我们的衣服可以御寒。 The thief stole all her jewelry. 小偷把她所有的首饰都偷走了。
