Corrosion resistance of new epoxy–siloxane hybrid coatings A laboratory study

Progress in Organic Coatings 69 (2010) 278–286
Contents lists available at ScienceDirect
Progress in Organic
Coatings
j o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /p o r g c o a
t
Corrosion resistance of new epoxy–siloxane hybrid coatings.A laboratory study
I.Díaz,B.Chico,D.de la Fuente,J.Simancas,J.M.Vega,M.Morcillo ?
Department of Materials Engineering,Degradation and Durability National Center for Metallurgical Research (CENIM-CSIC),Gregorio del Amo 8,28040Madrid,Spain
a r t i c l e i n f o Article history:
Received 6April 2010
Received in revised form 9June 2010Accepted 28June 2010Keywords:
Epoxy–siloxane Zinc-rich primers
Accelerated corrosion tests Anticorrosive protection
a b s t r a c t
Traditional multilayer epoxy/polyurethane type anticorrosive paint systems are widely employed in the protection of steel structures due to their high ef?ciency against atmospheric corrosion.However,the use of isocyanate in the curing process,and the high volatile organic compound (VOC)content of such systems,makes it necessary to search for new isocyanate-free paints.
Hybrid organic–inorganic coatings,such as epoxy–siloxane coatings,represent a step forward in the ?eld of paint coatings for atmospheric corrosion protection.These new isocyanate-free hybrids present low VOC levels –due to the high solid content associated with their low viscosity –along with good heat and UV radiation stability and excellent chemical resistance.With the new polysiloxane inorganic resins it is hoped to improve the anticorrosive behaviour of traditional organic binders.
This work assesses the anticorrosive performance of one-layer epoxy–siloxane coatings compared to traditional two-layer epoxy/polyurethane coatings,in paint systems with and without an epoxy or silicate-type zinc-rich primer.
The anticorrosive properties of these coatings applied on steel substrates have been evaluated using a wide range of experimental techniques,namely measurement of water vapour and oxygen permeabil-ity in free ?lms,3-year outdoor testing,accelerated corrosion testing (condensing humidity,Kesternich,salt fog and Prohesion),and electrochemical impedance spectroscopy (EIS).Hybrid epoxy–siloxane ?lms show the lowest oxygen and water vapour permeability,capacitance values,and the highest ionic resis-tance values,along with excellent behaviour in humidity and Kesternich tests,outperforming traditional epoxy/polyurethane paints,while the behaviour of both types of coatings is similar in the salt fog,Pro-hesion and short-term outdoor tests.This type of paint requires the presence of zinc-rich primers to optimise its anticorrosive behaviour.
? 2010 Elsevier B.V. All rights reserved.
1.Introduction
In recent years great technological advances have been seen in the ?eld of anticorrosive paints.One of the basic objectives of the paint industry today is to develop products that are less aggressive to worker health and the environment.In this context,the synthe-sis of new polymers and pigments and the development of surface preparation and paint application equipment using advanced tech-nologies is a substantial contribution to the attainment of these goals.
One such product already available on the international market is a new type of polysiloxane resin based paints.One of the char-acteristics of these paints is that their high solid content,which means a low organic solvent concentration,allowing high paint layer thicknesses using conventional application methods,which is a fundamental factor for reducing application costs and execu-tion times.The technical literature presents hybrid epoxy–siloxane
?Corresponding author.Tel.:+34915538900;fax:+34915347425.E-mail address:morcillo@cenim.csic.es (M.Morcillo).coatings as excellent protective coatings due to their chemical structure,which combines the durability and hardness of epoxy coatings and notably improves on the gloss and colour retention of urethane type coatings.
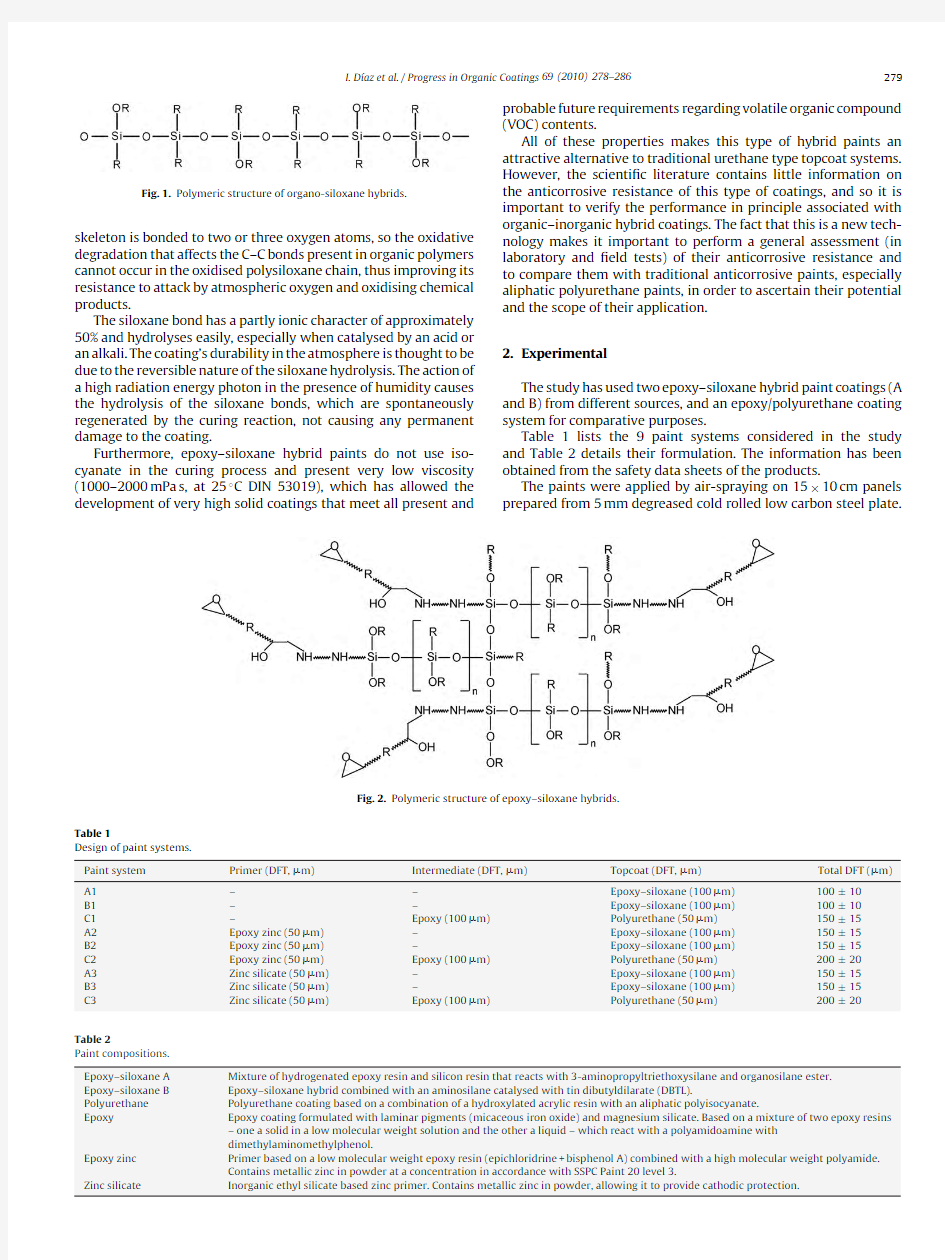
Fig.1depicts the general chemical structure of organo-siloxane hybrids [1],where the R group may be hydrogen,methyl,ethyl,propyl,octyl,pentyl,acrylic or other organic substituents.The selection of these substituents allows the design of hybrid organo-siloxane polymeric networks with different reactivities and different physical,chemical and anticorrosive properties [2].Fig.2depicts the chemical structure of the epoxy–siloxane hybrid [3,4].
Epoxy–siloxane polymers are formed by skeletons of repetitive –Si–O–units,chemically bonded to lateral epoxy organic chains.The siloxane bond –Si–O–has a binding energy of 445Kj/mol,which is higher than the 358Kj/mol of the –C–C–bond that forms the repetitive unit of the organic polymer coatings [5–7].Consequently a higher activation energy is required to break the siloxane inor-ganic polymer skeleton,which means that these coatings must provide greater durability and better resistance to atmospheric degradation,heat,chemical attack,and UV radiation than organic polymers.Furthermore,the silicon in the polysiloxane polymer
0300-9440/$–see front matter ? 2010 Elsevier B.V. All rights reserved.doi:10.1016/j.porgcoat.2010.06.007
I.Díaz et al./Progress in Organic Coatings69 (2010) 278–286
279
Fig.1.Polymeric structure of organo-siloxane hybrids.
skeleton is bonded to two or three oxygen atoms,so the oxidative degradation that affects the C–C bonds present in organic polymers cannot occur in the oxidised polysiloxane chain,thus improving its resistance to attack by atmospheric oxygen and oxidising chemical products.
The siloxane bond has a partly ionic character of approximately 50%and hydrolyses easily,especially when catalysed by an acid or an alkali.The coating’s durability in the atmosphere is thought to be due to the reversible nature of the siloxane hydrolysis.The action of a high radiation energy photon in the presence of humidity causes the hydrolysis of the siloxane bonds,which are spontaneously regenerated by the curing reaction,not causing any permanent damage to the coating.
Furthermore,epoxy–siloxane hybrid paints do not use iso-cyanate in the curing process and present very low viscosity (1000–2000mPa s,at25?C DIN53019),which has allowed the development of very high solid coatings that meet all present and probable future requirements regarding volatile organic compound (VOC)contents.
All of these properties makes this type of hybrid paints an attractive alternative to traditional urethane type topcoat systems. However,the scienti?c literature contains little information on the anticorrosive resistance of this type of coatings,and so it is important to verify the performance in principle associated with organic–inorganic hybrid coatings.The fact that this is a new tech-nology makes it important to perform a general assessment(in laboratory and?eld tests)of their anticorrosive resistance and to compare them with traditional anticorrosive paints,especially aliphatic polyurethane paints,in order to ascertain their potential and the scope of their application.
2.Experimental
The study has used two epoxy–siloxane hybrid paint coatings(A and B)from different sources,and an epoxy/polyurethane coating system for comparative purposes.
Table1lists the9paint systems considered in the study and Table2details their formulation.The information has been obtained from the safety data sheets of the products.
The paints were applied by air-spraying on15×10cm panels prepared from5mm degreased cold rolled low carbon steel
plate.
Fig.2.Polymeric structure of epoxy–siloxane hybrids.
Table1
Design of paint systems.
Paint system Primer(DFT,?m)Intermediate(DFT,?m)Topcoat(DFT,?m)Total DFT(?m)
A1––Epoxy–siloxane(100?m)100±10
B1––Epoxy–siloxane(100?m)100±10
C1–Epoxy(100?m)Polyurethane(50?m)150±15
A2Epoxy zinc(50?m)–Epoxy–siloxane(100?m)150±15
B2Epoxy zinc(50?m)–Epoxy–siloxane(100?m)150±15
C2Epoxy zinc(50?m)Epoxy(100?m)Polyurethane(50?m)200±20
A3Zinc silicate(50?m)–Epoxy–siloxane(100?m)150±15
B3Zinc silicate(50?m)–Epoxy–siloxane(100?m)150±15
C3Zinc silicate(50?m)Epoxy(100?m)Polyurethane(50?m)200±20
Table2
Paint compositions.
Epoxy–siloxane A Mixture of hydrogenated epoxy resin and silicon resin that reacts with3-aminopropyltriethoxysilane and organosilane ester.
Epoxy–siloxane B Epoxy–siloxane hybrid combined with an aminosilane catalysed with tin dibutyldilarate(DBTL).
Polyurethane Polyurethane coating based on a combination of a hydroxylated acrylic resin with an aliphatic polyisocyanate.
Epoxy Epoxy coating formulated with laminar pigments(micaceous iron oxide)and magnesium silicate.Based on a mixture of two epoxy resins –one a solid in a low molecular weight solution and the other a liquid–which react with a polyamidoamine with
dimethylaminomethylphenol.
Epoxy zinc Primer based on a low molecular weight epoxy resin(epichloridrine+bisphenol A)combined with a high molecular weight polyamide.
Contains metallic zinc in powder at a concentration in accordance with SSPC Paint20level3.
Zinc silicate Inorganic ethyl silicate based zinc primer.Contains metallic zinc in powder,allowing it to provide cathodic protection.
280I.Díaz et al./Progress in Organic Coatings69 (2010) 278–286 Prior to the application of the paint coatings the specimens were
shot-blasted with shot steel abrasive to Sa3grade according to ISO
8501-1[8],reaching an average roughness of6.3?m.
The panels were air dried for7days prior to testing.A scribe of
0.3mm in width and6cm in length was cut in the lower zone of the
panels to evaluate the inhibitive properties of the paint coatings in
that zone.
2.1.Permeability measurements
For the determination of water vapour and oxygen per-
meation rates through the paint coating[9,10],free?lms
with an area of11.34cm2of the coating material were used.
The thicknesses obtained for free?lms of the epoxy–siloxane
coatings were125±10?m,while those corresponding to the
epoxy/polyurethane coating were notably higher,210±10?m.
Water vapour permeability was ascertained by means of perme-
ability cups using a50%relative humidity gradient(ASTM D1653
[11]).The temperature was kept constant at25?C.
Oxygen permeability measurements were performed following
the electrochemical technique,using a selective oxygen electrode
[12,13].The free?lm,with an area of12.56cm2,was located in a
permeation cell between two compartments of0.027L each,one
with distilled water kept constantly saturated with oxygen and the
other with deaerated distilled water in which an Orion selective
oxygen electrode(model97-08)was placed.The water in both
compartments was maintained at25±1?C.Readings of the oxy-
gen transferred through the paint?lm were taken with an Orion
analyser-processor,model SA720.Permeabilities were calculated
according to the following expressions:
water permeability=
( m1×t)
s
,
oxygen permeability=
( m2×t×v)
s
where m1is the daily mass loss,in units(mg/day); m2 the amount of oxygen permeated per unit of time,in units (mg O2/min L);t the free?lm coating thickness;s the coating area; v is the volume of water in which the oxygen is dissolved.
2.2.Adhesion tests
The method used to evaluate the adhesion of all the paint sys-tems was the adhesion test,according to standard ASTM D4541-02 [14].The test was performed with an AT-1000portable adhesion meter,which measures the pulling force in units of kgf and applies a progressive pulling rate of not more than1MPa/https://www.360docs.net/doc/be7586541.html,e was made of a20mm diameter dolly?xed to the coating using a two-component epoxy glue,considering a tested area of3.14cm2.
Prior to the performance of the test the contact surface between the dolly and the different coatings was polished with a400-grain?ne abrasive.This serves to improve the contact between the two surfaces,preventing premature failure of the paint–glue joint. After cleaning the surfaces with ethanol,the glue was applied and allowed to dry for24h at room temperature.The coating was scored through to the substrate around the loading?xture with a circular hole cutter before running the test.
Two pieces of information are obtained from a pull-off test.The ?rst is the pull-off strength of the coating and the second is where the split occurred in the paint system.The split could be an adhesive break,a cohesive break,a combination of both,or a failure of the glue[15]
.
Fig.3.Equipment used in impedance measurements.
2.3.Corrosion tests
The paints were subjected to the following accelerated corrosion tests:humidity(ASTM D4585-07[16]);Kesternich(ASTM G87-02, 2L SO2[17]);salt spray(ASTM B117-09[18]);and Prohesion(ASTM G85-09[19]).
An atmospheric exposure test was also conducted on a corro-sion rack located on the roof of the CENIM laboratory in an urban atmosphere of corrosivity category C2–C3,for low carbon steel, according to ISO9223[20].After3years of exposure none of the tested coating systems(see Table1)had suffered deterioration due to blistering or rusting of the metallic substrate.
2.4.Electrochemical impedance spectroscopy(EIS)
The anticorrosive performance of paint systems A1,B1and C1was also monitored using electrochemical impedance spec-troscopy(EIS)in a classic three-electrode cell with a working area of9.62cm2.EIS measurements were performed using a potentio-stat/galvanostat(AutoLab EcoChemie PGSTAT30)equipped with a FRA2frequency response analyser module(Fig.3).Frequency scans were carried out by applying a±5mV amplitude sinusoidal wave perturbation,close to the corrosion potential.Five impedance-sampling points were recorded per frequency decade.The analysed frequency range was from100kHz to1mHz and the electrolyte used was a0.1M sodium sulphate solution.The impedance data was analysed using the electrochemical impedance software ZView?(Version2.9c,Scribner Associates,Inc.,USA).
3.Results and discussion
3.1.Permeability measurements
3.1.1.Water vapour permeability
Table3summarises the different water vapour permeability grades obtained with the traditional two-layer epoxy/polyurethane topcoat system(C1)and the two one-layer epoxy–siloxane hybrid coatings(A1and B1).It must be pointed out the higher water vapour
Table3
Water vapour permeability results.
Free coatings Thickness
(?m)
Slope
(mg/day)
Permeability
(mg mm/cm2day) Epoxy–siloxane(A1)125±107.99660.0957
Epoxy–siloxane(B1)125±1010.55300.1107
Epoxy/polyurethane(C1)210±109.08500.1734
I.Díaz et al./Progress in Organic Coatings69 (2010) 278–286281
Fig.4.Adhesive failure in system B2,between the intermediate epoxy coating and the epoxy–siloxane topcoat.Left:adhesion test.Right:test panel scribe zone in test panel.
Table4
Oxygen permeability results.
Free coatings Thickness
(?m)Slope
(mg O2/L min)
Permeability
(mg mm/cm2day)
Epoxy–siloxane(A1)125±100.01900.0085
Epoxy–siloxane(B1)125±100.01950.0083
Epoxy/polyurethane(C1)210±100.02020.0133 permeability of epoxy–siloxane B1in relation to epoxy–siloxane A1.
According to this data,the entry of electrolyte necessary for the corrosive process to the metallic substrate is delayed by the pres-ence of the protective one-layer epoxy–siloxane hybrid coatings (A1,B1)due to their considerably lower water vapour permeabil-ity,64%and55%respectively,than the conventional two-layer epoxy/polyurethane system(C1).
3.1.2.Oxygen permeability
Table4displays the permeability values obtained for the tested free?lms and the thickness of the latter.
The one-layer epoxy–siloxane free?lms(A1,B1)possess con-siderably lower oxygen permeability,64%and62%,respectively, than the two-layer epoxy/polyurethane?lm(C1).This hinders the corrosive process,polarising the cathodic reaction of atmospheric corrosion by limiting the access of oxygen to the cathodic areas of the metal.
3.2.Adhesion tests
Table5shows the pulling force of all the tested paint systems and the type of rupture with the coatings forming the paint systems.
Both the traditional epoxy/polyurethane coating systems and the epoxy–siloxane hybrid coatings present similar pulling forces. Adhesion values of epoxy–siloxane A are higher than the corre-sponding to epoxy–siloxane B.Most failures occur at the paint–glue interface during the pull-off test,thus indicating the great adhe-sion between the different coatings of the paint systems.A higher percentage of cohesive failure is observed in the interme-diate layer(epoxy)of the traditional coatings compared to the epoxy–siloxane hybrids.The only exception is the case of the epoxy zinc/epoxy–siloxane coating(B2),where the failure is completely adhesive between the topcoat and the primer,showing a lower pulling force than the rest of the paint systems[21–23](see Fig.4).
3.3.Accelerated corrosion tests
3.3.1.Humidity test
After5304h of permanent humidity exposure all the coat-ing systems,both the conventional epoxy/polyurethane and the epoxy–siloxane hybrid types,show very low degrees of rusting.The systems including epoxy–siloxane hybrid coatings,which as noted above have lower water vapour permeability rates,present consid-erably less blistering than the traditional systems[23],although the difference is smaller when the primer used in the hybrid systems
is epoxy zinc.Hybrid system B2is the only one that experiences slightly greater blistering than the traditional systems(see Table6),Fig.5.Evaluation of blistering degree for the different paint systems exposed in permanent humidity condensation conditions.
282I.Díaz et al./Progress in Organic Coatings69 (2010) 278–286
Table5
Results of adhesion test.
Paint system(Table1)Adhesion pull-off(MPa)Type of rupture
%Glue%Adhesive%Cohesive
Intermediate/topcoat Intermediate Topcoat
A19.8100–––
B17.7100–––
C18.172–28–
A27.2100–––
B2 2.3–100––
C29.428–72–
A37.57129––
B37.485–15–
C3 6.8100–––
Table6
Results obtained in permanent humidity condensation cabinet after5304h of exposure.
Paint system Rusting degree(ASTM D-610[24])Blistering degree Observation at scribe
ASTM D-714[25]Numerical scale[26]a
A19–10–10b Rusting
B110–10b Rusting
C19–10D31Rusting
A29–10D75Rusting
B210D20Rusting adhesion fail at intermediate/topcoat C29–10D42Very slight rusting
A310–10b Very slight rusting
B310–10b Very slight rusting
C39–10D53Absence of rusting
a The values of size and frequency of blisters have been converted,according to ASTM D-714,into numeric values using the Keane conversion table.
b6720h.
Table7
Kesternich resistance test after20exposure cycles.
Paint system Rusting degree(ASTM D-610[24])Blistering degree Delamination from scribe(mm)
ASTM D-714[25]Numerical scale[26]a
A110F89 1.5
B110F892
C110M663
A210F890.5
B210F890.75
C210M880.75
A310D860.5
B310F890.5
C310M440.5
a The values of size and frequency of blisters have been converted,according to ASTM D-714,into numeric values using the Keane conversion table.
as a consequence of the poor adhesion between the primer paint and the topcoat(see Table5and Fig.4).
With regard to the behaviour of the paint coatings in the scribe zone,the systems that include a silicate-type zinc-rich primer show a certain preponderance in terms of cathodic protection of the bare base steel.It is well known that inorganic ethyl silicate-type zinc-rich primers afford better and longer lasting cathodic protection than epoxy type zinc-rich primers,provided that a high metallic zinc content in the coating is assured[27].This is because wetting properties of the vehicle have a decisive in?uence on the elec-tronic conductive of the paint coating;the non-polar nature of ethyl silicate-type binder makes it dif?cult to wet zinc particles(“pseudo-
Table8
Resistance to salt fog after3456h of exposure.
Paint system Rusting degree(ASTM D-610[24])Blistering degree Delamination from scribe(mm)
ASTM D-714[25]Numerical scale[26]a
A17–1016
B12–1016.5
C18MD8715
A29–102
B29–103
C28–102
A38–10 1.25
B38–10 1.5
C39F89 1.5
a The values of size and frequency of blisters have been converted,according to ASTM D-714,into numeric values using the Keane conversion table.
I.Díaz et al./Progress in Organic Coatings 69 (2010) 278–286
283
Fig.6.Evolution of blistering degree for the different paint systems exposed in conditions of 2L SO 2in the Kesternich test.
wetting”vehicle),whereas epoxy type binder,being of polar nature,wet them relatively easily (“effective-wetting”vehicles)[28].
Fig.5displays the evolution of blistering for the different paint systems with exposure time,showing the excellent performance of the hybrid coatings compared to the traditional paint systems.Hybrid system B2is an exception to this behaviour.The traditional epoxy/polyurethane type systems start to deteriorate after 350h of
exposure.
Fig.7.Kesternich test.Aspect of the scribe zone.
3.3.2.Kesternich
After 20exposure cycles in the SO 2atmosphere (2L),no rusting is observed with any of the studied paint systems.How-ever,all the paint systems that include hybrid coatings,without exception,show notably less blistering than the conventional epoxy/polyurethane type systems (see Table 7),once again con-?rming the results obtained in the measurements of water vapour permeability (see Table 3).
Fig.6shows the evolution of blistering experienced by all the studied paint systems exposed to cycles of 2L SO 2.The paint coat-ings start to deteriorate due to blistering in SO 2atmospheres after approximately 10testing cycles.The absence of a zinc-rich primer leads to greater delamination of the coatings in the scribe zone (Fig.7).
3.3.3.Salt spray
The epoxy–siloxane coatings (without zinc-rich primer)show a considerable rusting degree,especially coating B1,which is greater than that of the conventional epoxy/polyurethane system (C1)(Table 8).However,the conventional system is the only one of all the studied coating systems to experience slight blistering after 3456h of exposure to the saline atmosphere.The one-layer hybrid systems (A1,B1)and the traditional two-layer system (C1)present notable rusting of the steel at the scribe and delamination as a con-sequence of the absence of zinc-rich primer.Here again it is possible to see the preponderance of the silicate-type zinc-rich primer over the epoxy type [27].
3.3.
4.Prohesion test
After 4896h of testing,all the paint systems without zinc-rich primer –both hybrids (A1,B1)and conventional (C1)–have a larger corroded area than the rest of the paint systems.This is most clearly seen in the case of hybrid system (B1),with a corroded area of 33%.On the other hand,except for the slight blistering experienced by the traditional paint system (C1),the coating systems do not present blistering (Table 9).
With regard to delamination at the scribe (Fig.8),the best results correspond to the paint system containing an epoxy zinc-rich
284I.Díaz et al./Progress in Organic Coatings69 (2010) 278–286
Table9
Resistance to Prohesion test after4896h of exposure.
Paint system Rusting degree(ASTM D-610[24])Blistering degree Delamination from scribe(mm)
ASTM D-714[25]Numerical scale[26]a
A18–1010
B12–1011
C18M88 5.5
A29–100.5
B29–10 4.5
C29–100.5
A39–1010Voluminous rust formations
B39–1026Voluminous rust formations
C39–109.5Voluminous rust formations
a The values of size and frequency of blisters have been converted,according to ASTM D-714,into numeric values using the Keane conversion table.
Table10
Prohesion test.Time(h)to formation of rust along entire length of scribe.
Paint system Time(h)for scribe fully rusted
A15
B15
C110
A2360
B2440
C2380
A31060
B31060
C31100
primer,in contrast to what happened in the condensing humidity and salt fog tests,where coating delamination in the scribe area was lower with the coatings applied on zinc silicate primer.This appar-ently anomalous result may be due to the strong attack of the ethyl silicate zinc-rich primer,and of the base steel once the cathodic protection capacity of this primer has been exhausted(Table10),Table11
Electrochemical parameters(C p,R p,/Z/at1mHz)of the tested coatings.
Paint systems C p(F)R p( )/Z/( )
Epoxy–siloxane(A1) 5.06×10?10–1011 Epoxy–siloxane(B1) 6.06×10?10 6.58×108109 Epoxy/polyurethane(C1)8.8×10?10 2.4×109109.5
leading to considerable lifting of the topcoat paint at the scribe. Observation of cross-sections of these paint systems in the scribe area(Fig.9)reveals strong attack of the zinc-rich primer and the presence of abundant voluminous rust at the scribe area in
the paint
system A3containing the zinc silicate primer(Fig.9top),in com-
parison with the relatively low attack at the scribe area in the paint
system A2containing the epoxy zinc primer(Fig.9down).EDS
spectra of the corrosion products reveals the presence of zinc from
the dissolution of zinc particles of the primer.
This strong attack may be due to the special microstructure of
the silicate-type zinc-rich primer’s with its pseudo-wetting vehicle, Fig.8.State of the different paint systems after4896h of exposure to Prohesion cyclic test.
I.Díaz et al./Progress in Organic Coatings69 (2010) 278–286
285
Fig.9.Cross-section optical micrographs(100×).Top:zinc silicate/epoxy–siloxane paint system(A3).Far from the scribe area(a),and at the scribe(b).EDS spectra(c)of the corrosion products.Down:epoxy zinc/epoxy–siloxane paint system(A2).At the scribe(d).
which facilitates the consumption of the zinc particles in its interior [27,28],and to the high corrosivity of the Harrison’s solution used in the Prohesion cyclic test[29],whose saline concentration in the scribe area also increases as a consequence of the successive drying stages in the different cycles.
3.4.Electrochemical impedance spectroscopy(EIS)
Only coatings A1,B1and C1were tested,i.e.the paint sys-tems without zinc-rich primer.Table11presents the resistance and capacitance values obtained by?tting of the respective impedance diagrams to equivalent circuits,and the impedance modulus values (/Z/)at the lowest measured frequency,1mHz,obtained from the Bode diagrams(Fig.10).
The models used are represented in Fig.10,where R e is the electrolyte resistance;R p is the electric resistance of the protective coating,and C p is the capacitance of the protective coating.These parameters may be taken as indicators of the state of the protective coating subjected to a medium that is aggressive to the metal.
The characteristic low frequency arc associated with the corro-sion process on the metallic surface,which can take place in pores or possible defects in the coating if the coating permeability allows diffusion of the electrolyte[30–32],is not observed in any of the three studied coatings(A1,B1or C1).
The epoxy–siloxane coating(A1)presents excellent barrier properties,acting as an almost perfect capacitor with a capacita-tive behaviour and an impedance modulus(/Z/)of the order of 1011 at a frequency of1mHz[33].Due to the absence of cor-rosive processes and diffusion processes on the metallic substrate, the impedance modulus for this coating at the lowest measured fre-quency,1mHz,may be taken as the ohmic or ionic resistance of the coating.
With regard to the epoxy–siloxane and epoxy/polyurethane coatings(B1and C1),the drop in R p may be associated to the pene-tration of electrolyte in the coating,slightly raising the capacitance compared to the epoxy–siloxane coating(A1)due to the increase in the dielectric constant of the medium.
This can be seen in the Nyquist diagram,which exhibits the characteristics of a semicircle in the Randles circuit(see Fig.10
). Fig.10.Nyquist and Bode diagrams,and associated equivalent circuits,of the tested coatings after504h of exposure to a0.1M Na2SO4solution.
286I.Díaz et al./Progress in Organic Coatings69 (2010) 278–286
However,all the studied coatings present an ionic resistance of more than107 ,a value above which coatings are considered to be in a good state and without deterioration[34].
Attention is drawn to the fact that a new time constant( )starts to appear at low frequencies for the epoxy–siloxane coating(B1), probably due to the charge transfer resistance of the metallic sub-strate or incipient diffusion controlled mass transport processes.
4.Conclusions
?Great differences in behaviour are seen between the two tested epoxy–siloxane formulations.While hybrid coating A equals or surpasses the protective capacity of hybrid coating B and the epoxy/polyurethane paint system in all the tests,hybrid coat-ing B frequently shows poorer anticorrosive properties than the conventional epoxy/polyurethane paint system.Differences in performance of the two epoxy–siloxane coatings could be attributed to the differences in water vapour permeabilities and adhesion values on steel base.
?The presence of zinc-rich primers generally optimises the good anticorrosive behaviour of epoxy–siloxane hybrid paints.?Epoxy–siloxane hybrid coating A presents excellent blistering behaviour,both with and without a zinc-rich primer(organic or inorganic),in the permanent humidity,Kesternich and salt fog tests,generally surpassing the performance of the conventional epoxy/polyurethane systems and proving to be highly suitable for environments with high humidity.
Acknowledgements
One of the authors,I.Díaz,would like to thank the State Agency Consejo Superior de Investigaciones Cientí?cas(CSIC)for the I3P programme contract granted,thanks to which it has been possible to undertake the work presented in this paper.
References
[1]H.Mayer,Surf.Coat.Int.2(1999)77.
[2]G.Reusmann,Macromol.Symp.187(2002)235.
[3]S.Ahmad,A.P.Gupta,E.Sharmin,M.Alam,S.K.Pandey,https://www.360docs.net/doc/be7586541.html,.Coat.54
(2005)248.
[4]J.-M.Yeh,H.-Y.Huang,C.-L.Chen,W.-F.Su,Y.-H.Yu,Surf.Coat.Technol.200
(2006)2753.
[5]J.M.Keijman,Properties and use of inorganic polysiloxane hybrid coatings for
the protective coatings industry,in:2as Jornadas da Revista Corrosao e Protec-cao de Materiais,Lisbon,November,2000.
[6]N.R.Mowrer,Performance Coatings and Finishes,Polysiloxanes,Ameron Inter-
national,2003,November.
[7]J.M.Keijman,High Solids Coatings:Experience in Europe and USA,in:Pro-
ceedings Protective Coatings Europe Conference,Paper40,The Hague,The Netherlands,March,1997.
[8]ISO8501-1,Preparation of steel substrates before application of paints and
related products,in:Visual Assessment of Surface Cleanliness.Part1.Rust Degrees and Preparation Grades of Uncoated Steel Substrates,ISO,Geneve, Switzerland,1988.
[9]H.Corti,R.Fernández-Prini,https://www.360docs.net/doc/be7586541.html,.Coat.10(1982)5.
[10]S.Guruviah,J.Oil Col.Chem.Assoc.53(1970)669.
[11]ASTM1653-72,Standard Test Method for Moisture Vapour Permeability of
Organic Coatings Films,ASTM,Philadelphia,1979.
[12]W.Funke,et al.,Proc.XV Fatipec Congress,Amsterdam,1980,p.111.
[13]S.Bechtle,Master’s dissertation.II.Institut für Technische Chemie der Univer-
sit?t Stuttgart,1985.
[14]ASTM D4541-02,Standard Test Method for Pull-off Strength of Coatings using
a Portable Adhesion Testers,ASTM,Philadelphia,2002.
[15]Ll.M.Smith,J.Protect.Coat.Linings18(2001)43.
[16]ASTM D4585-07,Testing Water Resistance of Coating Using Controlled Con-
densation,ASTM,Philadelphia,2007.
[17]ASTM G87-02,Standard Practice for Conducting Moist SO2Tests,ASTM,
Philadelphia,2007.
[18]ASTM B117-09,Standard Practice for Operating Salt Spray(Fog)Apparatus,
ASTM,Philadelphia,2009.
[19]ASTM G85-09,Annex A5:Standard Practice for Modi?ed Salt Spray(Fog)Test-
ing,ASTM,Philadelphia,2009.
[20]ISO9223,Corrosion of Metals and Alloys,Corrosivity of Atmospheres,ISO,
Geneve,1992.
[21]S.Ananda Kumar,M.Alagar,V.Mohan,Eur.Coat.J.5(1999)64.
[22]S.Ananda Kumar,M.Alagar,Eur.Coat.J.4(2001)152.
[23]A.Israel,Surf.Coat.Australia35(1998)24.
[24]ASTM D-610,Evaluating Degree of Rusting of Painted Steel Surfaces,ASTM,
Philadelphia,1968.
[25]ASTM D-714,Evaluating Degree of Blistering of Paints,ASTM,Philadelphia,
1987.
[26]J.D.Keane,J.A.Bruno,R.E.F.Weaver,Performance of alternate coatings
in the environment,Steel Structure Painting Council(SSPC),Pittsburgh, 1979.
[27]M.Morcillo,R.Barajas,J.M.Bastidas,J.Mater.Sci.25(1990)2441.
[28]K.M.Oesterle,Farbe Lack68(1962)753.
[29]I.Costa,S.E.Faidi,I.D.Scantlebury,Corros.Sci.35(1993)1367.
[30]S.Feliu,J.C.Galván,M.Morcillo,https://www.360docs.net/doc/be7586541.html,.Coat.17(1989)143.
[31]J.C.Galván,S.Feliu,M.Morcillo,https://www.360docs.net/doc/be7586541.html,.Coat.17(1989)135.
[32]S.Feliu,J.C.Galván,M.Morcillo,Corros.Sci.30(1990)989.
[33]D.Loveday,P.Peterson,B.Rodgers,J.Coat.Technol.1(2004)88.
[34]C.C.Lee,F.Mansfeld,Corros.Sci.41(1999)439.
环氧树脂用户手册
第一章工艺设备检查----------------------------------------------------------2 第二章装置吹扫------------------------------------------------------------------3 第三章公用工程系统投用------------------------------------------------------5 第四章装置水冲洗、水联运--------------------------------------------------7第五章装置气密------------------------------------------------------------------8第六章催化剂的装填------------------------------------------------------------9 第七章开工准备-----------------------------------------------------------------11 第八章开机流程----------------------------------------------------------------12 第九章装置事故预想方案----------------------------------------------------14 第十章设备的日常运行与维护----------------------------------------------16
环氧树脂使用说明
施工步骤要求 2、准备好必要的工具及养护品 3、确定修补区域,其修补处理范围应比实际破损范围向外扩大100mm,切割或剔凿出混凝土修补区域的垂直边缘,其深度≥5mm以免修补区域边缘薄片化。 4、将修补区域内混凝土基层表面浮尘、油污清理干净,并剔除疏松部分。 5、清理修补区域内裸露钢筋表面的锈质和杂物。 6、将清理好的修补区域内混凝土基层进行凿毛处理或用混凝土界面处理剂进行界面处理。 7、用气泵或水将处理过的修补区域内混凝土基层表面清扫干净,进行下道工序时不得有明水存留。 8、按推荐加水量10-20%(重量比)的配合比搅拌EC2000高强修补砂浆。采用机械搅拌2-3分名目即可并在利于搅拌的质量和速度。人工搅拌应在5分名目以保证搅拌均匀。 9、拌好的M由于MT-2000高强修补砂浆含有多种高分子聚合物改性外渗料及胶粉,使拌合好的MT-2000高强修补砂浆较粘稠,抹灰时应注意刀光洁。 10、对于表面需压光处理的,最外层抹灰应拌合略稀,并掌握好时间,以利于压光处理。 11、严禁在EC2000高强修补砂浆中掺入任何外加剂或外掺料。 12、使用温度为-5℃—40℃ 养护 1、夏季施工作业完毕后2-4小时,应及时浇喷水工喷洒养护剂或覆盖潮湿草袋进行养护,并保持表面湿润2-3天。 2、冬季施工完毕后,应立即覆盖塑料薄膜并加盖棉被进行保温养护。 3、 MT-2000高强修补砂浆为25kg袋装。 4、存放在通风干燥处并防止阳光直射。 5、保质期为6个月,超出保质期应复检,合格后方可使用。 ?1 环氧修补砂浆配比(重量比) ?2 环氧树脂:水泥:干砂=1:1.5~2:3~4 ?3
步骤: ?4 1.水泥以4 2.5级为宜,干砂过筛(02.5mm).所用砂子必须晒干或烘干。 ?5 2.将水泥与干砂按比例先混合均匀,摊开在铁板上。 ?6 3.将环氧树脂缓缓加入,先用木棒搅成散团,再用手搓成拳头大小团。 ?7 注:可根据部位不同,配置干稠或稀软不等的树脂砂浆。环氧树脂修补砂浆配比与步骤:?8 一。环氧树脂配比(重量比) ?9 树脂:二丁酯:乙二胺=1:0.25:0.07 ?10 步骤:1.称好树脂,按比例加入二丁酯匀,再按比例加入乙二胺 ?11 2.乙二胺有一定毒性,人站在上风口位置。
(完整版)环氧树脂主要性能指标的检测方法
三、环氧树脂主要性能指标的检测方法 1、环氧树脂环氧值、环氧当量的测定 可用光谱分析法或化学分析法进行分析,光谱分析比化学分析容易操作,但是需要用标准试祥做成定量线。 ①光谱分析法 用红外光谱、拉曼光谱或核磁共振光谱等分析方法是很普及的,可用于环氧树脂的定性分析或环氧基的定量分析。红外光谱吸收法:首先用一系列已知环氧当量的环氧树脂的红外光谱做出A910cm-1/A1610 cm-1 (其中910cm-1是环氧基的吸收峰,1610 cm-1是苯环的吸收峰)基线,然后做出A910cm-1/A1610 cm-1与环氧当量标准曲线。这样在测定某一环氧树脂试样的环氧当量时,只需知道该环氧树脂A910/M1610的比值,即可确定其环氧当量。 ②化学分析法 常用的化学分析方法是在适当的溶剂中,使用过量的盐酸与环氧基作用,定量生成氯醇,将过且的盐酸用碱滴定法定量,。常用的溶剂有丙酮、无水醚、吡啶等。有时不用盐酸,而用溴化化氢酸、碘化钾与盐酸、过氯酸与季铵溴化物等为卤化剂,进行直接滴定。 方法多种多样,现今国际上通用的分析法是高氯酸法,适用于各种环氧树脂,但操作过程繁琐。另外还有盐酸/丙酮法、盐酸吡啶法以及盐酸二氧六环法。我国沿用的测定方法以盐酸一丙酮法和盐酸一吡啶法,其中盐酸一丙酮法较适用于分子量在1500以下的环氧树脂,而
盐酸一吡啶法较适用于分子量在1500以上的环氧树脂。相对来说,盐酸一丙酮法应用较多。 溴化季按盐直接滴定法 a)原理 原理是通过高氯酸(HClO4)与溴化四乙基铵(NEt4Br)反应生成的溴化氢与1,2-环氧基的定量反应。该程序包括用高氯酸-冰醋酸标准溶液滴定溶解在含溴化四乙基铵的环氧树脂的二氯甲烷溶液,以结晶紫为指标剂,当环氧基被消耗完,过量的溴化氢会引起过量的结晶紫指标剂变色。 b)溶液配制 结晶紫指标剂:取结晶紫0.5g,溶解于100ml冰醋酸中即得, 0.1 mol /L高氯酸-冰醋酸标准溶液 配制取无水冰醋酸550ml,加入高氯酸HClO4(W/W在70%左右,比重1.75)8.2ml摇匀,在烧杯中缓缓滴加24ml醋酐,用玻璃棒不断搅拌,放冷至室温后,转移到1000ml容量瓶中,加无水冰醋酸稀释至刻度线,摇均匀后,放置24小时使醋酐与溶液中的水充分反应完全。即得0.1N浓度的HClO4-HAc标准溶液。 标定准确称取在105℃干燥至恒重的邻苯二甲酸氢钾KHC8H4O4约0.4g(准确至0.0001 g)置于锥形瓶中,加无水冰醋酸20ml,使溶解,加0.5%结晶紫冰醋酸溶液1—2滴,用高氯酸冰醋酸标准溶液滴定至蓝色,并将滴定结果用空白试验(即不加邻苯二甲酸氢钾)校正。计算如下:
环氧树脂的应用配方大全
环氧树脂的应用配方大全 一、粘合剂 配方一: 6101#环氧树脂100 691#甘油酯20-60铝粉15-20 160℃/2h+180℃/4h τ>36.6MPa 配方二:酚醛-环氧胶 酚醛树脂100 聚乙烯醇缩甲乙醛806101#环氧树脂302E4MZ 5 80℃/1h+130℃/4h 压力0.05MPa τ=23.3-27.8MPa τ50℃=7.2-7.6MPa 配方三:H703胶 618# 100环氧化聚丁二烯树脂20650#聚酰胺20600#双缩水甘油脂10 咪唑(100目)8β-羟基乙二胺8 压力0.07MPa,60℃/4h τ=30MPa τ100℃=19MPa 二、浇铸 在电子电气中,街 髦值缙 考 ⒋笮途 瞪璞福 美疵芊狻⒎莱钡取S没费跏髦 街 保 胗猛涯<粒 缂谆 柘鸾骸⒐栌秃蚉VC薄膜等,浇铸过程中要消除气泡,①加热驱赶气泡;②轻口倾注浇铸料;③最佳方法是浇铸好树脂后进行减压脱气泡。 配方一: 6101#环氧树脂100 聚壬二酸酐20纳迪克酸酐50 石英粉(>270目)200苄基二甲胺0.25 100℃/1h+120℃/1h +150℃/2h+180℃/4h+200℃/6h δ抗弯=113.8MPa,δ抗压=194MPa tgδ=8.5×10-3,ε=3.9Ω体积=9.4×1015Ω.cm 配方二: 634#环氧树脂100 铝粉(100-200目)170均苯四甲酸二酐21 顺丁烯二酸酐19 130℃/4h+160℃/12h+180℃/12h δ抗冲0.53MPa δ抗压=300MPa 三、玻璃钢 常用于环氧玻璃钢的环氧树脂,有普通双酚A型如681#、6101#、634#,酚醛型环氧树脂644#,脂环族环氧6207#和HY-201聚丁二烯环氧树脂。辅助材料中固化剂常用DTA、间苯二胺、顺丁烯二酸酐、邻苯二甲酸酐、内次甲基四氢邻苯二甲酸酐等,促进剂为三乙醇胺。 配方一: 6109#环氧树脂100苯乙烯5三乙醇胺6三乙烯四胺 4 室温10天,加上130℃6h τ=13MPa δ=298.5MPa δ抗压=300MPa 配方二: 644#酚醛环氧化100 NA酸酐68 二甲基苄胺1.8丙酮100 室温——120℃(40min)——200℃(40分) ——降温——卸模处理150℃/2h+260℃/1天 配方三: 634#环氧树脂323193#聚酯28邻苯二甲酸酐8BPO 2苯乙烯30 100。C/2h + 180。C/8h 弯曲强度和反弹能力佳。 四、涂料: 环氧涂料是环氧树脂应用最早的品种,其耐腐蚀性能超过醇酸树脂。目前,其最广泛应用的是环氧粉末涂料和水系涂料。 配方四:环氧呋喃防腐涂料 6101#环氧树脂100 呋喃树脂(2503#)15DBP 20 间苯二胺15 丙酮30-40 长石粉20
环氧树脂复合管说明书
里面红色的根据你是否要报通用管作适当修改。 煤矿井下用环氧树脂涂层供水排水 复合钢管 产 品 使 用 说 明 ××××管业有限公司
目录 一、概述------------------------------------------1 1、产品执行标准-----------------------------------1 2、产品特点---------------------------------------1 3、产品结构---------------------------------------1 4、主要用途及适用范围-----------------------------1 5、型号的组成及其代表意义-------------------------2 6、管材规格---------------------------------------2 二、产品技术特性----------------------------------3 三、管材的连接方法和安装要求----------------------3 四、使用与维护------------------------------------4 五、安全警示----------------------------------4 六、包装、运输及贮存------------------------------4 1、包装-------------------------------------------4 2、运输-------------------------------------------4 3、贮存-------------------------------------------4 七、售后服务--------------------------------------4 八、本公司联系方式--------------------------------5
水性丙烯酸涂料配方设计
1.丙烯酸酯涂料简介 1.1 定义 以丙烯酸酯或甲基丙烯酸酯为主要原料合成的树脂称丙烯酸酯树脂,由丙烯酸酯树脂为主要基料的涂料属丙烯酸酯涂料。 1.2 结构 丙烯酸树脂的化学结构如图1,其中R为-H、-CN、烷基、芳基和卤素等;R为-H、烷基、芳基、羟烷基;其中-COOR也被-CN、-CONH2、-CHO等基团取代。作为涂料用丙烯酸树脂则主要是丙烯酸、甲基丙烯酸及其脂与苯乙烯经共聚而得到的热塑性或热固性丙烯酸系树脂,以及其他树脂(如醇酸树脂、环氧树脂、聚氨酯树脂、聚酯树脂等)改性的丙烯酸树脂。 图1 1.3丙烯酸酯涂料的分类 1.3.1按成膜特性分类 (1)热塑性丙烯酸酯涂料 热塑性丙烯酸酯涂料由丙烯酸树脂溶于有机溶剂制得,如丙烯酸清漆、丙烯酸磁漆,带溶剂挥发后,形成美观而坚固的涂膜。 (2)热固性丙烯酸酯涂料 热固性丙烯酸酯涂料则是通过自交联或与环氧树脂、氨基树脂、
异氰酸酯等交联(常温或烘干)完成成膜过程,交联使漆膜变成巨大的网状结构,提高了涂膜多方面的物理性能及防腐蚀、耐化学品性能。 1.3.2按丙烯酸酯涂料形态分类 按丙烯酸酯聚合物的形态分类和性质分为三种:溶剂型、水性、无溶剂型,如表1-1。 表1-1 丙烯酸酯涂料按形态分类 1.3.3按丙烯酸酯涂料用途分类 ①木器用丙烯酸酯涂料;
②建筑用丙烯酸酯涂料; ③汽车用丙烯酸酯涂料; ④工业防腐蚀用丙烯酸酯涂料; ⑤塑料表面用丙烯酸酯涂料; ⑥家电用丙烯酸酯涂料; ⑦预涂装用丙烯酸酯涂料; 1.4热塑性丙烯酸树脂涂料的优点 ①与硝基清漆、醇酸树脂涂料相比,他的耐候性优良; ②保光性优良,具有深邃的光泽和透明性; ③耐水性优良,耐酸、耐碱性优良,对洗涤剂有较强的抗性; ④只要底漆选择适当,附着力就良好; ⑤抛光性良好; 1.5热塑性丙烯酸树脂涂料的缺点 ①施工性能不好,流动展平性不良,透干性不好,涂料易流挂; ②耐溶剂性差,当遇到溶剂时会发生再溶解容易溶胀; ③相溶性差,难以与其他树脂并用; ④热敏感性差,研磨性不好,糊砂纸。 2.水性丙烯酸酯树脂的合成 2.1合成原理
环氧树脂特性
环氧树脂 目录 材料简介 应用特性 类型分类 使用指南 国内主要厂商 环氧树脂应用领域 环氧树脂行业 材料简介 环氧树脂 是泛指分子中含有两个或两个以 上环氧基团的有机高分子化合 物,除个别外 ,它们 的 相对分子质量 都不高。 环氧树脂的 分子结构是以分子链中含有活泼 的环氧基团为其特征 ,环氧基 团 可以位于分子 链的末端、中间或成环状 结构。由于分子结构中 含有活泼的环氧基团,使 它们可与多 种类型的固化 剂发生交联反应而形成不溶、不 熔的具有三向网状结构的高聚 物。 应用特性 1 、 形式 多样。各种树脂、固化剂、改性剂体系几乎可以适应各种应用 对形式提出的要求,其 范围可以从极 低的粘度到高熔点固 体。 2 、 固化方便。选用各种不同的 固化剂,环氧树脂体系几乎可 以在 0 ~ 180 ℃温度范围内固化 。 3 、 粘 附力强。环氧树脂分子链中固有的极 性羟基和醚键的存在,使其对各种物质 具有很高的 粘附力。环氧 树脂固化时的收缩性低,产生的 内应力小,这也有助于提高 粘 附强度。 4 、 收缩 性低。 环氧树脂和所用的固化剂的反应是 通过直接加成反应或树脂分子中 环氧基的 开 环聚合反应来 进行的,没有水或其它挥发性副 产物放出。它们和不饱和聚 酯 树脂、酚醛树脂相比, 在固化过程中 显示出很低的收缩性(小于 2%)。 5 、 力学性能。固化后的环氧 树脂体系具有优良的力学性 能。 6 、 电性能 。固化后的环氧树脂体系是一 种具有高介电性能、耐表面漏电、耐电弧 的优良绝 缘 材 料。 7 、 化学 稳定性。通常,固化后的环氧树脂体系具有优良的耐 碱性、耐酸性和耐溶剂性。像固 化环氧体系的 其它性能一样, 化学 稳定性也取决于所选用的树脂和 固化剂。 适当地选用 环氧树脂 和 固化剂,可以 使其具有特殊的化学稳定性 能。 8 、 尺寸稳定性。上述的许多 性能的综合,使环氧树脂体系 具 有突出的尺寸稳定性和耐久性 。 9 、 耐霉菌。固化的环氧树脂 体系耐大多数霉菌,可以在苛 刻 的热带条件下使用。 类型分类 根据分子 结构,环氧树脂大体上可分为五 大类: 1 、 缩水甘油醚类环氧树脂 2 、 缩水甘油酯类环氧树脂 3 、 缩水甘油胺类环氧树脂 4 、 线型脂肪族类环氧树脂 5 、 脂环族类环氧树脂
环氧树脂
编辑本段类型 1、活性氢化物与环氧氯丙烷反应; 2、以过氧化氢或过酸(例如过醋酸)将双键进行液相氧化; 3、双键化合物的空气氧化; 4、由于它的性能并不是十分完美的,同时应用环氧树脂的对象也不是千 遍一律的,根据使用的对象不同,对环氧树脂的性能也有所要求,例如有的要求低温快干,有的要求绝缘性能优良。因而要有的放矢对环氧树脂加以改性。 编辑本段应用特性 1、形式多样。各种树脂、固化剂、改性剂体系几乎可以适应各种应用对 形式提出的要求,其范围可以从极低的粘度到高熔点固体。 2、固化方便。选用各种不同的固化剂,环氧树脂体系几乎可以在0~ 180℃温度范围内固化。 3、粘附力强。环氧树脂分子链中固有的极性羟基和醚键的存在,使 其对各种物质具有很高的粘附力。环氧树脂固化时的收缩性低,产生的内应力小,这也有助于提高粘附强度。 4、收缩性低。环氧树脂和所用的固化剂的反应是通过直接加成反应 或树脂分子中环氧基的开环聚合反应来进行的,没有水或其它挥发性副产物放出。它们和不饱和聚酯树脂、酚醛树脂相比,在固化过程中显示出很低的收缩性(小于2%)。 5、力学性能。固化后的环氧树脂体系具有优良的力学性能。 6、电性能。固化后的环氧树脂体系是一种具有高介电性能、耐表面 漏电、耐电弧的优良绝缘材料。 7、化学稳定性。通常,固化后的环氧树脂体系具有优良的耐碱性、 耐酸性和耐溶剂性。像固化环氧体系的其它性能一样,化学稳定性也取决于所选用的树脂和固化剂。适当地选用环氧树脂和固化剂,可以使其具有特殊的化学稳定性能。 8、尺寸稳定性。上述的许多性能的综合,使环氧树脂体系具有突出 的尺寸稳定性和耐久性。 9、耐霉菌。固化的环氧树脂体系耐大多数霉菌,可以在苛刻的热带 条件下使用。
环氧树脂化学品安全技术说明书_(MSDS)
环氧树脂化学品安全技术说明书 (MSDS) 一:标识 1. 化学品中文名称:环氧树脂 2. 化学品英文名称: Epoxy resin 3. 分子式: 4. 分子量: 5. 企业名称: 6. 地址: 7. 邮编:传真: 8. 企业应急电话: 9. 技术说明编码: 二:成分/组成信息 1. 主要成分:环氧树脂 2. 含量: 99% 3. CAS No. 24969-06-0 三:危险性概述 1. 危险性类别:第3.2类中闪点易燃液体 2. 侵入途径:吸入·食入·经皮吸收 3. 健康危害:接触危害主要为过敏而出现皮肤疾病,皮炎有时伴有眼睛上呼吸道刺激,制备和使用工人可有头痛,恶心,食欲不振,眼睑水肿等症 四:急救措施 1. 皮肤接触:脱去污染衣着,立即用大量流动清水冲洗至少15分钟,就医。 2. 眼睛接触:立即翻开上下眼睑,用流动清水或生理盐水冲洗至少15分钟,就医。 3. 吸入:呼吸道受剌激立即移至新鲜空气处,保持呼吸道通畅,必要时给与输氧,停止呼吸时,立即进行人工呼吸,就医。 4. 食入:误入口内立即用清水漱口并服大量冷开水催吐,有条件的可用牛奶洗胃 五:消防措施 1. 危险特性:粉体与空气形成爆炸性混合物,达到一定浓度,遇火星会发生爆炸。 2. 有害燃烧产物:二氧化碳·一氧化碳
3. 灭火方法:雾状水、泡沫、二氧化碳、干粉、砂土 六:泄漏应急处理 应急处理:切断火源。迅速撤离泄漏污染区人员至安全地带,并进行隔离,严格限制出入。建议应急处理人员戴自给正压式呼吸器,穿防护服。尽可能切断泄漏源。防止进入下水道、排洪沟等限制性空间。小量泄漏:尽可能将溢漏液收集在容器内,用砂土、活性碳或其它惰性材料吸收残液,也可以用不燃性分散剂制成的乳液刷洗,洗液稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容。转移至专用收集器内,回收或运至废物处理场所处理。七:操作处置与储存 1. 操作注意事项:密闭操作,加强通风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式面具(半面罩),戴化学安全防护眼镜,穿防渗透工作服,戴橡胶耐油手套。远离火种、热源、工作场所严禁吸烟。使用防爆型的通风系统和设备。防止蒸气泄漏到工作场所空气中。搬运时要轻装轻卸,防止包装及容器损坏。配备相应品种和数量的消防器材及泄漏应空气中浓度超标时,建议佩戴过滤式防毒面具(半面罩)。紧急事态抢救或撤离时,急处理设备。倒空的容器可能残留有害物。 2. 储存注意事项: 贮存于阴凉通风的专用库房内。避免与火种、热源接触,避免日光直晒。禁止与自燃品共贮共运。罐储时要有防火防爆技术措施,远离火种。注意轻搬轻放,防止容器损坏。勿在居民区和人口稠密区停留,储区应备有泄漏应急处理设备。 八:接触控制/个体防护 1.职业接触限值:未制定标准 2.监测方法: 3.工程控制:密闭操作,全面排风。 4.呼吸系统防护:佩带自吸过滤式防毒面具(半面罩) 5.眼睛防护:戴化学安全防护镜 6.身体防护:穿防静电工作服 7.手防护:操作人员应戴橡胶手套 8.其他防护:工作后,沐浴更衣。保持良好卫生习惯。工作中禁止吸烟、进食、饮水。九:理化特性 1. 外观与性状:根据分子结构的不同,其物态可从无臭、无味黄色透明液体至固态 2. 熔点(°C):145-155 3. 沸点(°C):
常用环氧树脂参数总结
常用环氧树脂参数总结 一、缩水甘油基型环氧树脂: 1.缩水甘油醚型环氧树脂 1.1双酚A型环氧树脂: 双酚A型环氧树脂是应用最广泛的树脂之一,占环氧树脂树脂总产量的90%。在分子结构中含有羟基和醚键,固化过程进一步生成新的—OH和—O—,使固化物具有很高的内聚力和粘附力。因此可以对金属、陶瓷、木材、水泥和塑料进行粘接。 另外,双酚A型环氧树脂属无毒树脂,其白鼠的最低口服致死量为LD50为11.4g/kg。 双酚A型环氧树脂的牌号与性质表 新牌号原牌号外观粘度(Pa.s)软化点(℃)环氧值 E—55 616# 浅黄粘稠液体 6-8 ---- 0.55-0.56 E—51 618# 浅黄粘稠液体 10-16 ---- 0.48-0.54 E—44 6101# 黄色高粘度液体 20-40 ---- 0.41-0.47 E—42 634# 同上---- 21-27 0.38-0.45 E—35 637# 同上---- 20-35 0.30-0.40 E—31 638# 浅黄粘稠液体---- 40-55 0.23-0.38 E—20 601# 黄色透明固体---- 64-76 0.18-0.22 E—14 603# 同上---- 78-85 0.10-0.18 E—12 604# 同上---- 85-95 0.10-0.18 E—06 607# 同上---- 110-135 0.04-0.07 E—03 609# 同上---- 135-155 0.02-0.04 E—01 665# 液体 30-40 ---- 0.01-0.03 1.2双酚S型环氧树脂 双酚S型环氧树脂是由双酚S和过量环氧氯丙烷在碱性条件下缩聚得到的耐高温环氧树脂。 双酚S为浅黄色固体,由东北石化研究所研制,全名为“4,4‘—二羟基二苯双缩水甘油醚环氧树脂”,胺类、酸酐、咪唑均能固化双酚S,其固化物具有热变形温度高、热稳定性能好的特点。这是因为分子中极性强的砜基—SO2—取代双酚A中的异丙基,提高了热稳定性;砜基改善了粘附力,增强了环氧基的开环活性。 1.3双酚F型环氧树脂 双酚F型环氧树脂是由双酚F和过量环氧氯丙烷(1:10),在四甲基氯化铵和NaOH条件下,经醚化和闭环反应,缩聚而成的。 双酚F型环氧树脂的粘度低,可用于碳纤维复合材料、玻纤增强塑料以及地下油井的灌封材料。 1.4环氧化线型酚醛树脂 环氧酚醛是由低分子量酚醛树脂与环氧氯丙烷在酸催化剂下缩合而成,兼有酚醛和双酚A型环氧树脂的优点。按线型酚醛树脂分子量和发羟基含量不同,可以合成不同分子量和官能度的环氧酚醛,如甲酚线型酚醛树脂。 环氧酚醛高粘度半固体,平均官能度为2.5-6.0,软化点≤28℃,环氧值0.53-0.57,在上海树脂厂和无锡树脂厂生产。为改善工艺,添加低粘度的稀释剂,或与双酚A混合使用。 胺类、酸酐类和咪唑均能固化环氧酚醛。在150℃以下固化环氧酚醛和双酚A型环氧树脂的热变形温度相近。例如: 固化剂固化条件用量% 热变形温度(℃)
HE环氧导电银胶使用说明书
H E环氧导电银胶使用 说明书 文件管理序列号:[K8UY-K9IO69-O6M243-OL889-F88688]
H20E环氧导电银胶使用说明书一.H20E是双组分,100%固含量银填充环氧树脂胶黏剂,专为导电粘接而 设计。由于该产品具有很高的热传导率,因此它也被广泛的应用于热处理 方面。H20E使用方便,可用于自动机械分配,丝网印刷,移印或手工操作。 H20E可耐受300°C到400°C的高温,并且耐湿性极佳,可达到JEDECⅢ 级、Ⅱ级的塑封耐湿要求。通泰化学。 二.外观、固化及性能 Ⅰ.银色,光滑的触变性膏状 Ⅱ.固化设备可选择烘箱、加热板、隧道炉等,最低固化温度条件为:175℃/45秒或150℃/5分钟或120℃/15分钟或80℃/3小时 Ⅲ.粘度: BROOKFIELD转子粘度计设置为100rpm/23℃时,2200-3200厘泊(cps) 操作时间:2.5天(通常可认为是胶黏剂粘度增加一倍所需要的时间) 保质期:-40℃低温隔绝水汽,六个月~一年 触变指数:3.69,(表示胶流变性能的参数,一般可认为触变指数越高, 胶的流动性越低,越易维持胶体原有形态。) 玻璃化温度:≥80℃ 硬度:ShoreD75 线性热膨胀系数:低于玻璃化温度时30×10-6in/in/℃ 高于玻璃化温度时158×10-6in/in/℃ 芯片粘接强度:>5kg(2mm×2mm)或1700psi 热分解温度:425℃(10%热重量损失)
连续工作温度:-55℃至200℃ 间歇工作温度:-55℃至300℃ 储能模量:808,700psi 填料粒径:≤45微米 体积电阻:≤0.0004欧姆-厘米 热导率:2.5W/mK 产品由树脂、银粉、固化剂、稳定剂等成分按化学反应配比混合成单一组分。银粉和树脂、固化剂的密度差异比较悬殊,在液态状况下,容易导致沉淀,一般针筒包装H20E 产品在解冻后需要在48小时内使用完毕,故针筒包装产品均根据使用量定单针筒包装含量。
环氧树脂基本知识
环氧树脂及环氧树脂胶粘剂的基本知识 (一)、环氧树脂的概念: 环氧树脂是指高分子链结构中含有两个或两个以上环氧基团的高分子化合物的总称,属于热固性树脂,代表性树脂是双酚A型环氧树脂。 (二).环氧树脂的特点(通常指双酚A型环氧树脂) 1.单独的环氧树脂应用价值很低,它需要与固化剂配合使用才有实用价值。 2.高粘接强度:在合成胶粘剂中环氧树脂胶的胶接强度居前列。3.固化收缩率小,在胶粘剂中环氧树脂胶的收缩率最小,这也是环氧树脂胶固化胶接高的原因之一。例如: 酚醛树脂胶:8—10% ;有机硅树脂胶:6—8% 聚酯树脂胶:4—8% ;环氧树脂胶:1—3% 若经过改性加工后的环氧树脂胶收缩率可降为0.1—0.3%,热膨胀系数为6.0×10-5/℃ 4.耐化学性能工好:在固化体系中的醚基、苯环和脂肪羟基不易受酸碱侵蚀。在海水、石油、煤油、10%H2SO4、10%HCl、10%HAc、10%NH3、10%H3PO4和30%Na2CO3中可以用两年;而在50%H2SO4和10%HNO3常温浸泡半年;10%NaOH(100℃)浸泡
一个月,性能保持不变。 5.电绝缘性优良:环氧树脂的击穿电压可大于35kv/mm 6.工艺性能良好、制品尺寸稳定、耐性良好和吸水率低。 双酚A型环氧树脂的优点固然好,但也有其缺点: ①.操作粘度大,这在施工方面显的有些不方便 ②.固化物性脆,伸长率小。 ③.剥离强度低。 ④.耐机械冲击和热冲击差。 (三).环氧树脂的应用与发展 1.环氧树脂的发展史: 环氧树脂是1938年由P.Castam申请瑞士专利,由汽巴公司在1946年研制出最早的环氧粘接剂,1949年美国的S.O.Creentee研制了环氧涂料,我国于1958年开始环氧树脂的工业化生产。 2.环氧树脂的应用: ①涂料工业:环氧树脂在涂料工业中需用量最大,目前较广泛使用的有水性涂料、粉末涂料和高固分涂料。可广泛用于管道容器、汽车、船舶、航天、电子、玩具、工艺品等行业。 ②电子电器工业:环氧树脂胶可用于电气绝缘材料,例如整流器、变压器的密封灌注;电子元器件的密封保护;机电产品的绝缘处理与粘
环氧树脂添加剂[仅供参考]
环氧树脂添加剂 一、稀释剂 稀释剂主要作用是降低环氧树脂配方体系的粘度,改善工艺性能。但稀释剂的加入对环氧树脂固化物的HDT、机械性能等有很明显的影响。 1.非活性稀释剂 在此物理混入过程中,不能参与固化反应,仅起到稀释粘度作用,其用量约5—20%为宜。 非活性稀释剂大部分是高沸点溶剂如邻苯二甲酸二丁酯、邻苯二甲酸二辛酯等。其中邻苯二甲酸二丁酯作为良好的增韧剂和稀释剂使用,加17份二丁酯,双酚A,环氧树脂粘度从15.0降至4.0 Pa.s,二乙烯三胺固化后HDT下降20℃左右。 环氧树脂常用的溶剂和稀释剂如表 2.活性稀释剂 主要是含有环氧基团的低分子环氧化合物,能与环氧树脂固化反应。其加入对固化物性能影响不大,可分为单环氧基和双环氧基活性稀释剂。 2.1单环氧活性剂 A.苯基缩水甘油醚:690#,粘度为7厘泊,上海树脂厂生产 B.丙烯基缩水甘油醚:500#,粘度为2厘泊,上海树脂厂生产 C.丁基缩水甘油醚:501#(稀释剂),粘度为2厘泊,粘度低,毒性小,其用量为树脂量10—15%,上海树脂厂生产 D.对甲苯酚缩水甘油醚 E.乙烯基环己烯甘油醚 F.甲基丙烯酸缩水甘油酯 某些单环氧稀释剂如690#,500#和501#对胺类固化剂反应活性较大;而烯烃或脂环族单环氧稀释剂对酸酐固化剂反应活性较大。 2.2 双环氧稀释剂 A.双缩水甘油醚:600#,粘度为4—6厘泊,无锡树脂厂生产 B.乙二醇双缩水甘油醚:512#,粘度为100厘泊,上海树脂厂生产 C.甘油环氧:662#,粘度为300厘泊,上海树脂厂生产 D.间苯二酚双缩水甘油醚:680#,粘度为200—600厘泊,上海新华树脂厂生产E.丁二烯环氧 F.异氰酸三缩水甘油酯 二、增韧剂:
环氧树脂910AB浇封(胶粘)使用工艺说明书
环氧树脂910A/B浇封(胶粘)使用工艺说明书 The Instruction of Process of 910A/B Encapsulation(Adhesion) of Epoxy Resin 1.主题内容与适用范围(Subject and Applicable Scope) 本说明书规定了本公司防爆电器产品、防爆灯具等使用环氧树脂910A/B浇封(胶粘)时的基本要求、操作程序、工艺参数及产品检验。 It is stipulated that the basic requirements, operational procedures, process parameters and product Inspection of 910A/B encapsulation (adhesion) of epoxy resin which is used for encapsulation (adhesion) in our explosion-proof electric apparatus, explosion-proof light fittings and so on. 2. 基本要求(Basic Requirement) 2.1 贮存(Storage) 在通风、干燥、阴凛、密封条件下贮存,保存期限为6个月。 It shall be stored in ventilated, dry, dark and sealed place, with the storage life of six months. 2 2 施工环境(Construction Environment) 施工环境应清洁、明亮。 The construction environment shall be clear and bright。 2.3 浇封(胶粘)的表面要求The Requirement of the-Encapsulated(adhered) Surface 将与胶接触面的油污、飞边、毛刺、漆皮等附着物进行清除;用干净布块擦拭浇封(胶粘)面;浇封(胶粘)件的表面,无污垢,无灰尘等方能进行浇封(胶粘):对受潮或流入液体的浇封(胶粘)件应放置在65±2℃电烘箱进行干燥处理,干燥时间不少于30分钟。Remove the attachments such as grease, flash, burr, etc. from the contact area with the adhesive; Wipe the encapsulated (adhered) surface with clean cloth; Only performing encapsulation (adhesion) when on the surface of encapsulated piece there is no grease, dust etc.. For the encapsulated(adhered) pieces with the dampness or liquid, they should be placed in the electric oven at 65℃more or less within 2℃for dryness for no less than 30 minutes. 3 操作程序及工艺参数( Operational Procedures and Process Parameters) 环氧树脂910A/B的使用工艺流程示意如下: The applied process flow chart of 910A/B encapsulation (adhesion) of epoxy resin is as follows:
环氧树脂MSDS
化学品安全技术说明书 第一部分化学品及企业标识 化学品中文名称:环氧树脂 化学品俗名或商品名: 化学品英文名称:Epoxy resin 企业名称:中国石化集团资产经营管理有限公司巴陵石化分公司地址:湖南岳阳云溪区 邮编:414014 电子邮件地址: 传真号码:(国家或地区代码)(区号)(电话号码) 企业应急电话:(国家或地区代码)(区号)(电话号码) 技术说明书编码: 生效日期:年月日 国家应急电话: 第二部分成分/组成信息 纯品 √混合物□ 化学品名称:环氧树脂
有害物成分浓 度CAS No. 环氧树脂99% 24969-06-0 第三部分危险性概述 危险性类别:第3.2类中闪点易燃液体 侵入途径:吸入·食入·经皮吸收 健康危害:接触危害主要为过敏而出现皮肤疾病,皮炎有时伴有眼睛上呼吸道刺激,制备和使用工人可有头痛,恶心,食欲不振,眼睑水肿等症 环境危害:无资料 燃爆危险:粉体与空气形成爆炸性混合物,达到一定浓度,遇火星会发生爆炸 第四部分急救措施 皮肤接触:脱去污染衣着,立即用大量流动清水冲洗至少15分钟,就医。 眼睛接触:立即翻开上下眼睑,用流动清水或生理盐水冲洗至少15分钟,就医。吸入:呼吸道受剌激立即移至新鲜空气处,保持呼吸道通畅,必要时给与输氧,停止呼吸时,立即进行人工呼吸,就医。 食入:误入口内立即用清水漱口并服大量冷开水催吐,有条件的可用牛奶洗胃 第五部分消防措施 危险特性:粉体与空气形成爆炸性混合物,达到一定浓度,遇火星会发生爆炸。有害燃烧产物:二氧化碳·一氧化碳 灭火方法及灭火剂:雾状水、泡沫、二氧化碳、干粉、砂土 灭火注意事项:喷水冷却容器,可能的话将容器从火场移至空旷处
环氧树脂胶粘剂的常用配方
环氧树脂胶粘剂的常用配方 玻璃钢 常用于环氧玻璃钢的环氧树脂,有普通双酚A型如681#、6101#、634#,酚醛型环氧树脂644#,脂环族环氧6207#和HY-201聚丁二烯环氧树脂。辅助材料中固化剂常用DTA、间苯二胺、顺丁烯二酸酐、邻苯二甲酸酐、内次甲基四氢邻苯二甲酸酐等,促进剂为三乙醇胺。 配方一: 6109#环氧树脂 100 苯乙烯 5 三乙醇胺 6 三乙烯四胺 4 室温10天,加上130℃6h τ=13MPa δ=298.5MPa δ抗压=300MPa 配方二: 644#酚醛环氧化 100 NA酸酐 68 二甲基苄胺 1.8 丙酮 100 室温——120℃(40min)——200℃(40分) ——降温——卸模处理150℃/2h+260℃/1天 配方三: 634#环氧树脂 32 3193#聚酯 28 邻苯二甲酸酐 8 BPO 2 苯乙烯 30 100。C/2h + 180。C/8h 弯曲强度和反弹能力佳。 配方一: 618# 100 DTA 8 DBP 20 AL2O3(200目) 100 固化条件:压力(MPa)/温度℃/时间(h)0.05/20℃/24h τ=18MPa 适用金属玻璃和陶瓷粘接。 配方二: 618# 100 二乙基丙胺 8 DBP 20 AL2O3 100 0.05/20℃/48h τ >20MPa 用途同上。 配方三:HYJ-6# 618#100 DBP 15 AL2O3 25 2#SiO22-5 四乙烯五胺 12 0.05/20℃/48h AL/玻钢>20MPa 适用于金属/玻璃钢粘接。 配方四: 618# 100 间苯二胺 18 600#稀释剂10 间苯二酚 10 0.05/20℃/24h τ=17.5MPa τ200℃=5.0MPa 用于耐热接头粘接。 配方五:913# A组:601#环氧 600#稀释剂201#聚酯铝粉和石英粉 B组:BF3乙醚四氢呋喃 A3PO4 A:B=10:1 0.05/15℃/6h τ=19MPa 低温快速固化适用于寒冷地区。 配方六: 四氢呋喃聚醚环氧 5 590#固化剂KH-550 0.2 0.05/30℃/30h τ
涂料配方设计
1,介绍: 粉末涂料由于其具有的无溶剂、施工简单、利用率高等特点而在全球市场高速增长,有机硅耐高温粉末涂料在美国八十年代在烤炉方面首先得到应用,而在九十年代中期快速在欧美市场快速增长。随着中国逐渐成为全球的灶具、烤炉等主要的生产基地。市场对耐高温粉末涂料的需要也日益增长。本文对耐高温粉末涂料的配方设计、问题处理、生产工艺等进行了介绍。 2,原理及性能介绍 2.1 原理 有机硅树脂的反应机理都是非常类似,其自身可以交联。在高温下的固化反应式如下: ~Si - OH + HO - Si ~ - - - > ~Si - O - Si ~ + H2O ~Si - OR + HO - Si ~ - - - > ~Si - O - Si ~ +ROH 此外,有机硅树脂中侧基不同的有机基团的热稳定性也有所不同:苯基〉甲基〉乙基〉丙基〉丁基〉己基 通常,有机硅树脂的固化温度不能低于200度。而270 和 350 °C之间的温度范围对于有机硅耐高温粉末涂料来说是个比较敏感的范围点。因为在此时有机硅组分还没有完全烧结完成,而有机组分已经开始燃烧分解。 此外,由于低Tg的有机硅树脂在储存和生产运输过程中遇到的结块问题使开发高Tg(玻璃化温度)的有机硅树脂也成为必然。现在,德国瓦克化学公司已经推出了Tg 〉65 的应用于耐高温粉末涂料的有机硅树脂,成功解决了高温天气下的运输、储存问题。 2.2 有机硅粉末涂料应该具有的性能? 与有机树脂不同的是,与适当的颜、填料配合使用的有机硅树脂应具有优秀的长期耐热性(200 - 650 °C)。 此外,对于食品接触的场合,有机硅树脂还应符合FDA 175.300 and BGVV – XV。良好的冷热交变性。通过把热板直接浸入冷水中,而涂膜不会损坏。 3.配方设计 3.1 基料的选择: 有机硅树脂是耐高温粉末涂料的必不可少的基料,有机硅树脂可以单独作为基料或与聚酯、环氧树脂拼用提高涂膜的耐高温性。同时配方中也应选用耐高温的无机颜料与填料以及适当的助剂。目前用于耐高温粉末涂料的有机硅树脂主要分为以下两种:
环氧树脂简介
环氧树脂简介 环氧树脂是一种分子内含有两个或两个以上的环氧基,并以脂肪族、脂环族或芳香碳键为骨架并能通过环氧基团反应形成热固性树脂的低聚物。它具有良好的粘接性、电绝缘性、低收缩性、化学稳定性、耐高低温性、耐磨性等优异性能。环氧树脂通常作为胶粘剂、涂料和复合材料等的树脂基体,广泛应用于建筑、机械、电子电气、航空航天等领域。全球各品种占环氧树脂总量的比例顺次为:双酚A型环氧树脂、阻燃溴化环氧树脂、酚醛型环氧树脂、脂环族环氧树脂等。固化后的环氧树脂具有良好的物理化学性能,它对金属和非金属材料的表面具有优异的粘接强度,介电性能良好,变定收缩率小,制品尺寸稳定性好,硬度高,柔韧性较好,对碱及大部分溶剂稳定,因而广泛应用于国防、国民经济各部门,作浇注、浸渍、层压料、粘接剂、涂料等用途。 环氧树脂使用时必须加入固化剂,优良的固化剂能赋予环氧树脂固化产物具有优异性能,通过固化反应,使环氧树脂生成立体网状结构的产物,成为具有真正使用价值的环氧树脂材料。开发新型固化剂远比开发新型环氧树脂更为重要。 1环氧固化剂的分类 (1) 按酸碱性质分为碱性和酸性两类 碱性固化剂:包括脂肪二胺、多胺、芳香族多胺、双氰双胺、咪唑类、改性胺类。 酸性固化剂:包括有机酸酐、三氟化硼及其络合物。 (2) 按固化机理分为加成型和催化型 加成型固化剂:包括脂肪胺类、芳香族、脂肪环类、改性胺类、酸酐类、低分子聚酰胺和潜伏性胺; 催化型固化剂:包括三级胺类和咪唑类。 (3) 根据多元分类法分为显在型和潜伏型 显在型固化剂:芳香胺、脂环胺、聚酰胺、酸酐、酚醛、聚硫醇及催化型; 潜伏型固化剂:双氰胺、有机酸酰肼及酮亚胺微胶囊。 2固化剂改性研究 目前,有关环氧树脂固化剂的研究内容主要是改善环氧树脂的脆性、耐温性、耐候性、固化速度等方面的缺陷,提高其性能。固化剂改性的主要方法是通过化学反应在原有固化剂结构中引入新的官能团或特殊结构以及合成新的固化剂品种。 (1)多元胺类固化剂改性研究
环氧树脂的固化
实验五 环氧树脂的固化 化工系 毕啸天 2010011811 一、实验目的 1.了解高分子化学反应的基本原理及特点 2.了解环氧树脂的制备及固化反应的原理、特点 二、实验原理 热固性树脂是一类重要的树脂材料,环氧树脂(epoxy resins )就是其中的一大品种。含有环氧基团的低聚物,与固化剂反应形成三维网状的固化物,是这类树脂的总称,其中以双酚A 型环氧树脂产量最大,用途最广。它是由环氧氯丙烷与双酚A 在氢氧化钠作用下聚合而成。根据不同的原料配比,不同反应条件,可以制备不同软化点、不同分子量的环氧树脂。其通式如下: CH 2 CH CH 2 O C CH 3 CH 3 OCH 2CHCH 2 OH n C CH 3CH 3 OCH 2 CH CH 2 O 环氧树脂通常用下面几个参数表征: 1.树脂粘度 2.环氧当量或环氧值 3.平均分子量和分子量分布 4.熔点或软化点 环氧值是表征环氧树脂质量的重要指标。它表示每100g 环氧树脂中含环氧基的摩尔数。我国环氧树脂部颁牌号中的两位数字是该牌号树脂的平均环氧值×100,所以部颁牌号可以很简明的表示出该环氧树脂的主要特征。 环氧树脂的结构中末端的活泼的环氧基和侧羟基赋予树脂反应活性,双酚A 骨架提供强韧性和耐热性;亚甲基链赋予树脂柔韧性;羟基和醚键的高度极性,使环氧树脂分子与相邻界面产生了较强的分子间作用力。双酚A 型环氧树脂综合性能好,因而用途广泛,商业上称作“万能胶”。 环氧树脂在未固化前呈热塑性的线性结构,通过与固化剂发生化学反应,形成网状结构的大分子,才具有使用价值。环氧树脂固化物的性能除了取决于自身的结构特性以外,还取决于固化剂的种类。此外固化物性能还受固化反应程度的影响。采用的固化条件不同,交联密度也会不同,所得固化物的性能也各异。环氧树脂的固化剂种类很多,不同的固化剂,其交联反应也不同。 未固化的环氧树脂是粘性液体或脆性固体,没有实用价值,只有与固化剂进行固化生成交联网络结构才能实现最终用途。环氧树脂与固化剂的反应,除了一般的脂肪胺和部分脂环胺类固化剂可以在常温固化外,其它大部分脂环族胺和芳香胺类以及全部的酸酐类固化剂都需要在较高的温度下经过较长的时间才能发生固化交联反应。为了降低固化温度,使用促进剂是必要的,适用于胺类和酸酐类固化环氧树脂的促进剂可分为亲核型、亲电型和金属羧酸(或乙酰丙酮)盐三类。环氧树脂的固化反应是通过环氧基的开环反应完成的,末端基为环氧基的树脂可以和多种含活泼氢的化合物反应。活泼氢对环氧化合物的作用先是在环氧基的 氧原子上引起质子的亲电附加,生成H 3O +离子,此反应非常迅速,在此H 3O + 离子的作用下进行亲核进攻,使环氧基开环。含有活泼氢的化合物有醇、酚、羧酸、硫醇、酰胺、脲类和异氰酸酯等,上述反应并不需要消除小分子就能使链增长或交联,因此环氧树脂比其它类型
