常用气体的物理化学特性

kmol
气体常数密度相对密度容积
R
ρ
s
(m3/kmol )(J/(kg·K ))(kg/Nm3)(空气=1)
1氢H 2 2.016022.427041250.08990.06952一氧化碳CO
28.010422.3984297 1.25060.96713甲烷CH 416.043022.3621
5180.71740.55484乙炔 C 2H 226.0380319 1.17090.90575乙烯C 2H 428.054022.2567296 1.26050.97486乙烷C 2H 630.070022.1872276 1.3553 1.0487丙烯C 3H 642.081021.9900197 1.9136 1.4798丙烷C 3H 844.097021.9362188 2.0102 1.5549丁烯C 4H 856.018021.6067148 2.5968 2.00810正丁烯n-C 4H 1058.124021.5036143 2.7030 2.09011异丁烯i-C 4H 1058.124021.5977143 2.6912 2.08112戊烯C 5H 1070.135021.2177118 3.3055 2.55613正戊烯C 5H 1272.151020.8910115 3.4537 2.67114苯C 6H 678.114020.3609106 3.8365 2.96715硫化氢
H 2S
34.07622.1802244 1.5363 1.18816二氧化碳CO 244.009822.2601188 1.9711 1.528917二氧化硫SO 264.05921.8821129 2.9275 2.26418氧O 231.998822.3923259 1.4291 1.105219氮N 228.013422.4035296 1.25040.967020空气28.96622.4003287 1.2931 1.000021水蒸气
H 2O
18.0154
21.629461
0.833
0.644
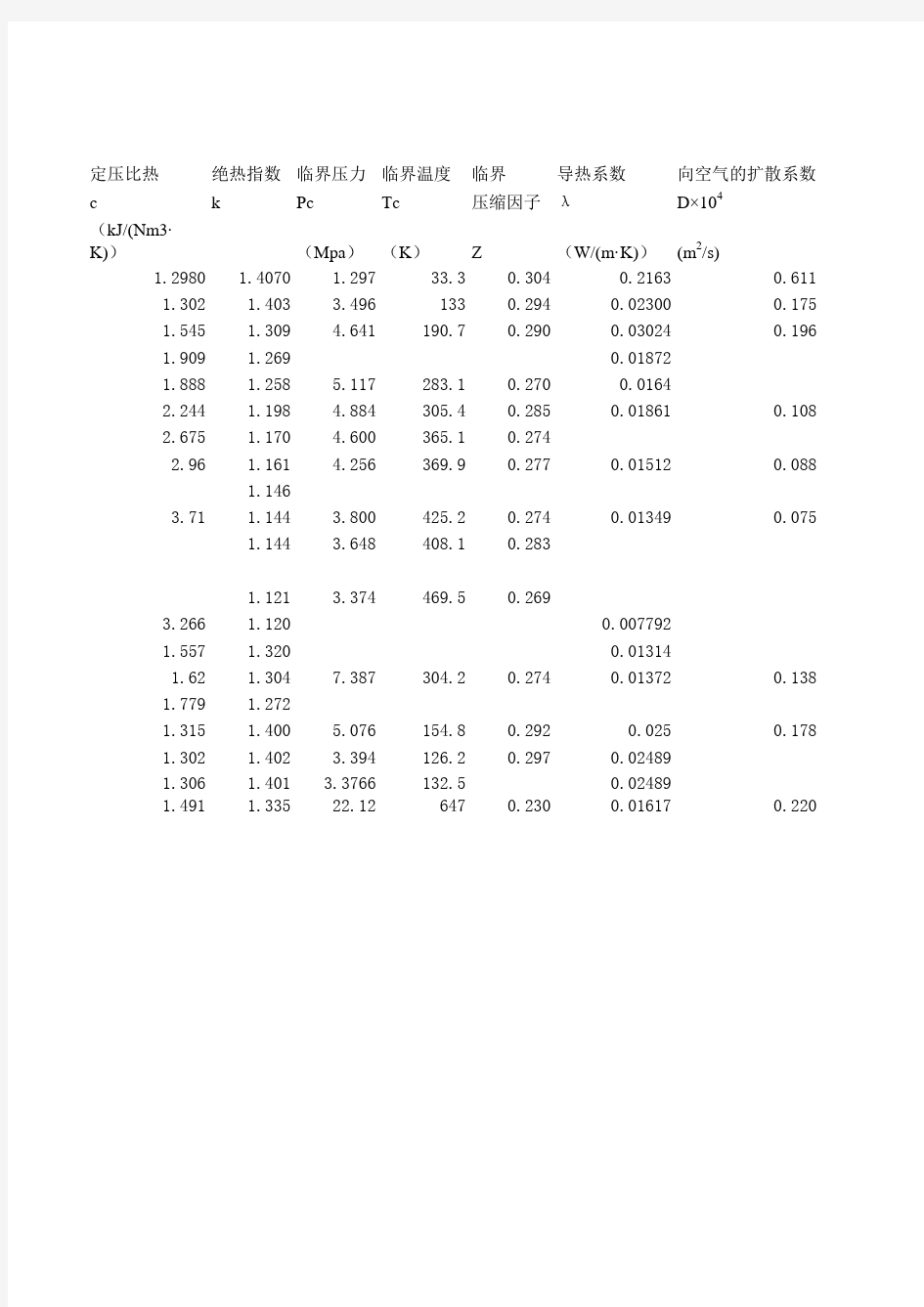
一些常用气体的物理化学特性(0℃、0.101325MPa )
序号气体
分子式分子量
定压比热绝热指数临界压力临界温度临界导热系数向空气的扩散系数c k Pc Tc压缩因子λD×104
(kJ/(Nm3·K))(Mpa)(K)Z(W/(m·K))(m2/s)
1.2980 1.4070 1.29733.30.3040.21630.611
1.302 1.403 3.4961330.2940.023000.175
1.545 1.309 4.641190.70.2900.030240.196
1.909 1.2690.01872
1.888 1.258 5.117283.10.2700.0164
2.244 1.198 4.884305.40.2850.018610.108
2.675 1.170 4.600365.10.274
2.96 1.161 4.256369.90.2770.015120.088
1.146
3.71 1.144 3.800425.20.2740.013490.075
1.144 3.648408.10.283
1.121 3.374469.50.269
3.266 1.1200.007792
1.557 1.3200.01314
1.62 1.3047.387304.20.2740.013720.138
1.779 1.272
1.315 1.400 5.076154.80.2920.0250.178
1.302 1.402 3.394126.20.2970.02489
1.306 1.401 3.376613
2.50.02489
1.491 1.3352
2.126470.2300.016170.220
运动粘度动力粘度常数最低着火温度ν×106
μ×106
C
(m 2/s)(kg·s/m 2)
(℃)高
93.000.852
90400H 2+0.5O 2=H 2O 28601313.30 1.690104605CO+0.5O 2=CO 228320814.50 1.060190540CH 4+2O 2=CO 2+2H 2O 8909438.0500.960198335C 2H 2+2.5O 2=2CO 2+H 2O 7.460.950257425C 2H 4+3O 2=2CO 2+2H 2O 14119316.410.877287515C 2H 6+3.5O 2=2CO 2+3H 2O 15608983.990.780322460C 3H 6+4.5O 2=3CO 2+3H 2O 20598303.810.765324450C 3H 8+5O 2=3CO 2+4H 2022214872.810.747385C 4H 8+6O 2=4CO 2+4H 2O 27191342.530.697349
365C 4H 10+6.5O 2=4CO 2+5H 2O 2879057460C 4H 10+6.5O 2=4CO 2+5H 2O 28735351.990.669290C 5H 10+7.5O 2=5CO 2+5H 2O 33780991.850.648260C 5H 12+8O 2=5CO 2+6H 2O
35384531.820.712380560C 6H 6+7.5O 2=6CO 2+3H 2O 33037507.63 1.190331270H 2S+1.5O 2=SO 2+H 2O
562572
7.09 1.4302664.14 1.23041613.60 1.98013113.30 1.70011213.40 1.75011610.12
0.860
673
(kj/kmol )热效应燃烧反应式
CO 2
低
高
低
空气
氧
2420641275310794 2.380.5 1.02832081264412644 2.380.5 1.080293239842359069.52 2.0 2.0585025648811.90 2.5 2.013213545634385948214.28 3.0 2.01428792703516439716.66 3.5 3.01927808936718766721.42 4.5 3.020454241012709324423.80 5.0 4.0254300412584711769528.56 6.0 4.0265889413388512364930.94 6.5 4.0265343911304812285730.94 6.5 5.0315796915921114883735.707.5 5.0327430816937715673338.088.0 6.0317161416225915577035.707.5 1.0
518644
25364
23383
7.14
1.5
理论烟
Nm 3/Nm 3干
kj/kmol )
热效应理论空气需要量,耗氧量(Nm 3/Nm 3干燃气)热值(kj/Nm 3)
燃烧热量温度(℃)H 2O
N 2
V f 0
下
上
1.0 1.88
2.88 4.075.922101.88 2.8812.574.223702.07.5210.52 5.015.020431.09.4012.40 2.580.026202.011.2815.28 2.734.02343
3.013.1618.16 2.913.021153.016.9222.92 2.011.72224
4.018.802
5.80 2.19.52155
4.022.5630.56 1.610.0
5.024.4434.44 1.58.521305.024.4434.44 1.88.521185.028.2038.20 1.48.7
6.030.0841.08 1.48.33.028.203
7.20 1.2
8.022581.0
5.64
7.64
4.3
45.5
1900爆炸极限(%)常压,20℃理论烟气量(Nm 3/Nm 3干燃气)
