盐雾测试报告-英文
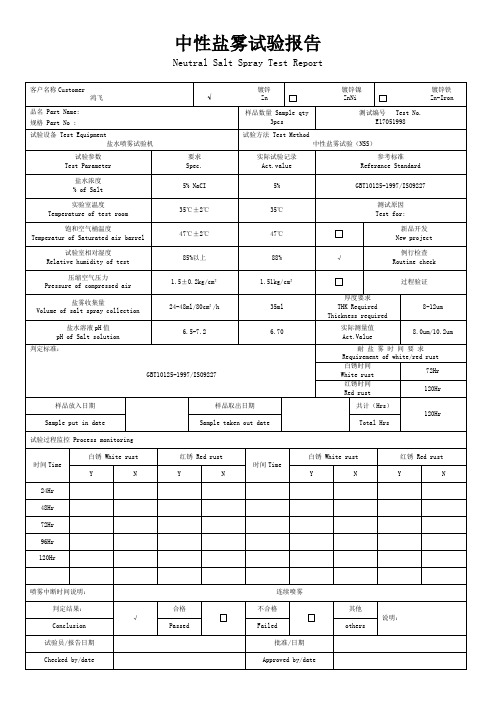
中性盐雾试验报告
6.5-7.2
6.70
实际测量值
Act.Value
8.0um/10.2um
判定标准:
GBT10125-1997/ISO9227
耐 盐 雾 时 间 要 求
Requirement of white/red rust
白锈时间
White rust
72Hr
红锈时间
Red rust
中性盐雾试验报告
Neutral Salt Spray Test Report
客户名称Customer
鸿飞
镀锌
√Zn
镀锌镍
ZnNi
镀锌铁
Zn-Iron
品名 Part Name:
规格 Part No :
样品数量 Sample qty
3pcs
测试编号 Test No.
E17051998
试验设备 Test Equipment
120Hr
样品放入日期
样品取出日期
共计(Hrs)
120Hr
Sample put in date
Sample taken out date
Total Hrs
试验过程监控 Process monitoring
时间Time
白锈 White rust
红锈 Red rust
时间Time
白锈 White rust
盐水喷雾试验机
试验方法 Test Method
中性盐雾试验(NSS)
试验参数
Test Parameter
要求
Spec.
实际试验记录
Act.value
参考标准
Referance Standard
JAG(中英文)盐雾测试报告2013-11-29-2

泰丰金属制品厂盐雾测试报告报告编号:2013.11.29-2名称Sample 45米四圆臂臂杆45M 4r/arm tube样品数量Sample Qty1pcs样品自编号Sample ref#275#表面处理Surface镀白锌Zinc plated样品状态Sample state良好well样品回厂时间Income time2013.10.28试验起始时间Time begins 2013.11.29PM3:00试验终止时间Time ends2013.11.30AM8:00供应商名称Supplier矮岗电镀厂盐水浓度Salt solution concentration (5±0.1)%所用盐为无水氯化钠,其碘化钠含量不得大于0.1%,总杂质含量不大于0.3%,盐溶液PH值6.5-7.2(5±0.1)% Sodium Chloride solution, PH index 6.5-7.2参考标准ReferenceGB/T 10125-1997 《人造气氛腐蚀试验-盐雾试验》National standard观察时间Time 项目Subject要求Requirement检查结果Result阶段图片Picture for each stage5小时5 hrs实验室温度Room temp.35±1℃34℃压力桶温度Inside temp.47±1℃47℃压缩空气压力Pressure1.0±0.01kgf/cm² 1.0 kgf/cm²喷雾量Spay volume1-2ml/80 cm²/h 1.5 ml/80 cm²/h 盐溶液PH值Solution PH6.5-7.2 6.8试件外观Outlook表面无锈蚀现象No rust表面少许白斑Small amountwhite spots观察时间Time 项目Subject要求Requirement检查结果Result阶段图片Picture for each stage10小时10hrs实验室温度Room temp.35±1℃36℃压力桶温度Inside temp47±1℃47℃压缩空气压力Pressure1.0±0.01kgf/cm² 1.0 kgf/cm²喷雾量Spay volume1-2ml/80 cm²/h 1.5 ml/80 cm²/h 盐溶液PH值Solution PH6.5-7.2 6.8试件外观Outlook表面无锈蚀现象No rust白斑变大White spotarea spread泰丰金属制品厂盐雾测试报告报告编号:2013.11.29-2说明:此表结果判断依据《泰丰金属制品厂盐雾试验方法和判定标准》第十、十一条执行。
英语盐雾实验报告

Date: [Insert Date]Test Conducted By: [Insert Name]Department: [Insert Department]Introduction:The salt spray test, also known as the salt fog test, is a method used to determine the corrosion resistance of materials under the influence of a salt spray environment. This test is commonly employed in the automotive, aerospace, and other industries to assess the durability and reliability of materials under harsh conditions. The present report outlines the procedures, results, and conclusions of the salt spray test conducted on [Insert Material/Component Name].Materials and Equipment:- Test samples: [Insert Material/Component Name]- Salt spray test chamber- Distilled water- Sodium chloride (NaCl) pellets- pH test strips- Magnifying glass- Weighing scale- Data recording sheetTest Procedure:1. Preparation of the salt spray solution:- Dissolve 50g of sodium chloride in 1 liter of distilled water.- Adjust the pH of the solution to 6.5 ± 0.5 using 5M sodium hydroxide or hydrochloric acid.- Ensure that the solution is free from impurities and properly mixed.2. Sample preparation:- Clean the test samples thoroughly to remove any surface contaminants.- Dry the samples at room temperature for at least 24 hours.- Weigh the samples and record their initial weights.3. Salt spray test:- Place the samples in the salt spray test chamber, ensuring that they are evenly spaced.- Adjust the temperature of the test chamber to 35 ± 2°C.- Set the relative humidity to 95 ± 5%.- Maintain the salt spray condition for the required duration (e.g., 24, 48, or 96 hours).4. Post-test evaluation:- Remove the samples from the test chamber and rinse them with distilled water to remove any residual salt spray.- Dry the samples at room temperature for at least 24 hours.- Inspect the samples visually using a magnifying glass for any signs of corrosion.- Record the observations on the data recording sheet.Results:- The test samples were exposed to salt spray for [Insert Duration] hours.- During the test, the pH of the salt spray solution was maintained at 6.5 ± 0.5.- The temperature and relative humidity of the test chamber were within the specified limits.- Post-test evaluation revealed the following observations:Sample A:- Initial weight: [Insert Weight]- Final weight: [Insert Weight]- Corrosion rating: [Insert Rating (e.g., 1 = No corrosion, 5 = Severe corrosion)]Sample B:- Initial weight: [Insert Weight]- Final weight: [Insert Weight]- Corrosion rating: [Insert Rating]Sample C:- Initial weight: [Insert Weight]- Final weight: [Insert Weight]- Corrosion rating: [Insert Rating]Conclusions:Based on the results of the salt spray test, the following conclusions can be drawn:1. The [Insert Material/Component Name] exhibited good corrosion resistance under the test conditions.2. The test duration of [Insert Duration] hours was sufficient to evaluate the corrosion resistance of the material.3. The pH of the salt spray solution was within the specified range, ensuring accurate test results.4. The temperature and relative humidity of the test chamber were maintained within the required limits, ensuring reliable results.Recommendations:- Further research is recommended to investigate the effects of different salt spray durations and intensities on the corrosion resistance of the material.- The test procedure can be modified to include other environmental factors, such as temperature and humidity, to simulate real-world conditions more accurately.- Additional tests, such as accelerated corrosion tests, can be conducted to validate the findings of the salt spray test.[End of Report]。
英文盐雾试验报告

1.Purpose Metal base will be corroded by water and oxygen (such as perspiration, salt mist, etc.) to cause rusting or blistering. This test accelerates such conditions to evaluate the corrosion resistance in a short time. 2.Sample size 1pcs test samples. 3.Test procedure TEST TOOLS a Salt water spray tester. b Salt water of 5 mass % (Use primary sodium oxide and deionized water.) c Clear adhesive tape Nichiban No.405. TEST METHOD One testing cycle is constituted of spraying at 35 ± 2 ° C for 8 hours, and storage under standard recovery condition for 12 hours. Carry out 2 testing cycles. 1 After the test, the specimen should be washed in running tap water thoroughly, and be dried. 2 The specimen should be inspected for any changes on the surface. 3 Put an adhesive tape firmly on the specimen, and remove it very quickly maintaining at right angles with the surface. ASSESSMENT Determination of adhesion with Cross-cut method and determination of scratch resistance with pencil hardness method or nail test (depending of the test objects). No: 20110504-001
盐雾测试报告 中英文对照全面

1. Test diagram & test method 测试图示和方法2. Test result 测试结果5PCS/2014-9-21Salt spray test machine DJS-EN61盐雾试验机:DJS-GU-358Test environment 测试环境Drawing NO.工程图号HDMI M/M Ni Plated 30AWG OD:5.0 L=1.5/2.0M Black/Sample Q'ty 样品数量Part/No.产品料号Test machine 测试设备温度:28℃/湿度:75%SHENZHEN EAST-TOPTECH ELECTRONIC TECHNOLOGY CO.,LTD深圳市东景盛电子技术有限公司Salt spray Test Report 盐 雾 测 试 报 告Product name 产品名称Product spec.产品规格HDMI AM TO AM 30AWG 1.4REV. OD :5.0mm Length :1.5/2.0MCustomer 客户名称/Test date 测试日期Note 备注According to GB/T10125-1997OK Sample No.OK Test result description 结果描述Judgement 综合判定2#3#4#Test Item 测试项目Salt spray test 盐水喷雾试验Result of measurement 测 试 结 果Test Requirement 测 试 要 求6.8Reference 参考6.5~7.235±2℃35±2℃1.0-2.0ml(80cm 2/H)5#■ PASS 合格 □ NG 不合格After ( 24 ) hours, test surface has no red phenomenon such as corrosion, bubble, crack After ( 24 ) hours, test surface has no red phenomenon such as corrosion, bubble, crack After ( 24 ) hours, test surface has no red phenomenon such as corrosion, bubble, crackOK OK OKJudgement 判定35℃35℃1.00KG/CM 225°GB/T10125-19971.5ml 24HAfter the test, take out the sample on the interior, natural drying 0.5 1 h, and then use clean flow of temperature is not higher than 35 ℃ water gently cleaning to remove the sample surface residual salt spray solution, then blow dry.试验结束后,取出试样放在室内,自然干燥0.5-1H,然后用温度不高于35℃的清洁流动水轻轻清洗以除去试样表面残留的盐雾溶液,再用吹风机吹干。
盐雾试验测试报告
5.Surface with no red rust after 96 hours.
检测设备 Test Equipment
盐雾试验机型号 Salt spray testenginery modelywx-250
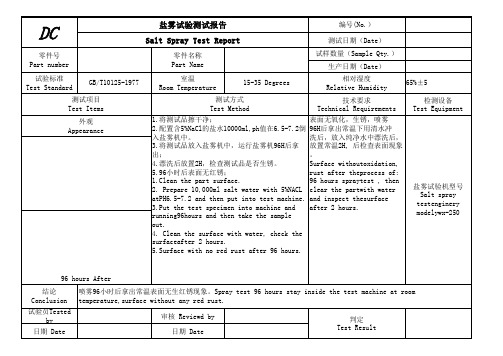
96 hours After
结论
喷雾96小时后拿出常温表面无生红锈现象。Spray test 96 hours stay inside the test machine at room
DC
盐雾试验测试报告 Salt Spray Test Report
编号(No.) 测试日期(Date)
零件号 Part number
零件名称 Part Name
试样数量(Sample Qty.) 生产日期(Date)
试验标准 Test Standard
GB/T10125-1977
室温 Room Temperature
96 hours spraytest , then
2. Prepare 10,000ml salt water with 5%NACL clear the partwith water
atPH6.5-7.2 and then put into test machine. and inspect thesurface
3.Put the test specimen into machine and after 2 hours.
running96hours and then take the sample
out.
4. Clean the surface with water, check the
surfaceafter 2 hrature,surface without any red rust.
盐雾测试 英文版

Salt spray testingSCOPE: this standard prescribles the conditions required in salt spray testing for specification purpose. It dose not prescribe the type of the specimen or sxposure periods to be used for a specific product, nor the interpretation to be given to the results.APPARATUS: 2.1 the apparatus required for salt spray testing consists of a fog chamber, a salt solution reservoir, a supply of suitably conditioned compressed air, one or more atomizing nozzles, specimen supports, provision for heating the chamber, and necessary means of control. The size and detailed the conditions obtained meet the requirements of this method. 2.2. drop of solution which accumulate on the celling or cover of the chamber shall not be permitted to fall on the specimens being tested. 2.3. drops of solution which fall from the specimens shall not be returned to the solution reservoir for respraying. 2.4. materials of construction shall be such that they shall not affect the correctiveness of the fog, nor be themselves corroded by the fog.TEST SPECIMENS: the type and number of test specimens to be used, as well as the criteria for the evaluation of the test results, shall be defined in the specifications covering the material or product being tested of shall be natually agreed upon by the purchaser and the supplier.PREPARATION OF TEST SPECIMENS 4.1. matallic and metallic-coated specimens shall be suitably cleared. The clearing method shall be optional depending on the nature of the surface and the contaminants, except that it shall not include the use of abrasives other than a parts of pure maggssium oxide nor of soivents which are cerretive or will deposit either corrosive or protective films. The ues of a nitric acid solution for the chemical cleaning, or passivation, of stainless steel specimens is permissible when agreed upon by the purchaser and the supplier. Care shall be taken that specimens are not recontaminated after caleaning by excessivs or careless handling. 4.2. specimens for evalustion of paints and other organic coasting shall be prepared according to applicable specification for the material being tested, or as agreed upon by the purchaser and aupplier. Otherwise the specimens shall consist of steel meetion the requirements of the methods for preparation of steel panels for testion paint, varnish, lacquer, and relate products and shall be cleaned and prepared for coation according to procedure A OF ASTM D609. 4.3. specimens coated with paints of nonmetallic coations shall not be cleaned of handled excessively prior to test. Whenever it is desired to determine the development of corrision from an abraded area in the paint of organic coation, a scratch of scribed line shall be made through the coation with a sharp instrument so as to expose the underlying metal before testing, the conditions of making the scratch shall be agreed upon between the purchaser and supplier. 4.5. unless otherwise specified, the cut edges of plated, coated, or duplex materials and areas containing identification marks or in contact with the racks of supports, shall be protected with a suitable coating stable under the conditions of the test, such as ceresin wax.POSITION OF SPECIMENS DURING TEST the position of the specimens in the salt spray chamber during the test shall be such that the following conditions are met. 5.1.unless otherwise specified, the specimens shall be supported or suspended between 15 and 30 degrees from the vertical and preferably parallel to the principle direction of horizontal flow of fog through the chamber, bassed upon the dominant surface being tested. 5.2. each specimen shall not contact each other or any metallic material of any material capable of acting as a wick. .5.3. each specimens shall be so placed as to permit free setting of fog on all specimens.TEST SOLUSION the salt solution shall be prepared by dissolving 5g of salt per every95ml of distilled water of water containing not more than 200 ppm of total solids. The salt used shall be sodium chloride substantially free of nickel and copper and containing on the dry basis not more than 0.1 percent of sodium iodide and not more than 0.3 percent of total impurities.CONDITIONS IN THE TEST CHAMBER 8.1. TEMPERARTURE the exposure zone of the salt chamber shall be maintained at 35 plus 1.1 or minus 1.7℃. the temperature within the exposure zone of the clossed cabinet shall be recorded at least twice a day at least 7 hours apart. 8.1. ATOMIZATION AND QUANTITY OF FOG at least two clean fog collectors shall be so placed within the exposure zone that no drops of solution from the test specimens of any other source shall be collected, the collectors shall be placed in the proximity of the test specimens, one nearest to any nozzle and the other farthest from all nozzles. The fog shall be such that for each 80 C㎡horizontal collection area there will be collected in each collector from 1.0 to 2.0 mL of solution per hour based on an average run of at least 16 hours. The sodiun chloride concentration of the collected solution shall be 5±1 percen mass. The pH of the collected solution shall be 6.5 to 7.2. the pH measurement shall be made electrometrically of colorimetrically using Bromthymol blue as the indicator. 8.3. the nozzle of nozzles shall be so directed or baffled that none of the spray can impinge directly on the test specimens. 8.4.dilution and evsporation of condensate should be avoided.CONTINUIY OF TEST unless otherwise specified in the specifications covering the material of product being tested, the test shall be continuous for the duration of the entire test period. Continuous operation implies that the chamber be clossed and the spray operation continuously except for the short daily interruptions necessary recordings as described in section8. operations shall be so scheduled that these interruptions are held to a minimum. PERIOD OF TEST. The period of test shall be as decignated by the specification covering the material of product being tested, or as mutually agreed by the purchaser and supplier. CLEANING OF TESTED SPECIMENS unless otherwise specified in the specification covering the material of product being tested, specimens shall be treated aws follows at the end of the test. 11.1 the specimens shall be carefully removed from the chamber. 11.2 specimens shall be gently rinsed in clean running warm water to remove salt deposits fromtheir surface, and then immediately dried. Drying shall be accoraplished with a stream of clean, compredded air at a gage pressure of 240 to 25 kPa.CVALUATION OF RESULTS a carefully and immediate examinatin shall be made for the extent of corrosion of the dry test specimens of for other failure as required by the specification covering the material or product being tested or by agreement between the purchaser and supplier.RECORDS AND REPORTS the following information shall be recorded, unless otherwise prescribed in the specification covering the material of product being tested. 13.1 type of salt and water used in preparing the salt solution. 13.2 all readings of temperature within the exposure zone of the chamber. 13.3 daily records of data obtained from each fog collecting device, including the following. 13.4 volume of salt solution collected in millilitres per hour per 80 C㎡13.5 concentration or specific gavity at 35℃of colution collected. 13.6 pH of collected solution. 13.7 type of specimen and dimension thereof or part number of description of part. 13.8. method of cleaning specimens before and ater testing. 13.9 method of supporting of suspending article in the salt apray chamber. 13.10. description of protection used as required in section 4. 13.11 exposure period. 13.12 inerruptions in test, cause and length of time. 13.13 results of all inspections.。
盐雾测试报告 中英文对照全面

1. Test diagram & test method 测试图示和方法2. Test result 测试结果5PCS/2014-9-21Salt spray test machine DJS-EN61盐雾试验机:DJS-GU-358Test environment 测试环境Drawing NO.工程图号HDMI M/M Ni Plated 30AWG OD:5.0 L=1.5/2.0M Black/Sample Q'ty 样品数量Part/No.产品料号Test machine 测试设备温度:28℃/湿度:75%SHENZHEN EAST-TOPTECH ELECTRONIC TECHNOLOGY CO.,LTD深圳市东景盛电子技术有限公司Salt spray Test Report 盐 雾 测 试 报 告Product name 产品名称Product spec.产品规格HDMI AM TO AM 30AWG 1.4REV. OD :5.0mm Length :1.5/2.0MCustomer 客户名称/Test date 测试日期Note 备注According to GB/T10125-1997OK Sample No.OK Test result description 结果描述Judgement 综合判定2#3#4#Test Item 测试项目Salt spray test 盐水喷雾试验Result of measurement 测 试 结 果Test Requirement 测 试 要 求6.8Reference 参考6.5~7.235±2℃35±2℃1.0-2.0ml(80cm 2/H)5#■ PASS 合格 □ NG 不合格After ( 24 ) hours, test surface has no red phenomenon such as corrosion, bubble, crack After ( 24 ) hours, test surface has no red phenomenon such as corrosion, bubble, crack After ( 24 ) hours, test surface has no red phenomenon such as corrosion, bubble, crackOK OK OKJudgement 判定35℃35℃1.00KG/CM 225°GB/T10125-19971.5ml 24HAfter the test, take out the sample on the interior, natural drying 0.5 1 h, and then use clean flow of temperature is not higher than 35 ℃ water gently cleaning to remove the sample surface residual salt spray solution, then blow dry.试验结束后,取出试样放在室内,自然干燥0.5-1H,然后用温度不高于35℃的清洁流动水轻轻清洗以除去试样表面残留的盐雾溶液,再用吹风机吹干。
中英文盐雾测试报告

TEST RECORD测试记录
START TIME开始时间:
END TIME完成时间:
TEST TEMPERATURE _100_℃TEST DEGREE
TEST INSTRUMENT TYPE 测试仪器 :
TEST CONDITION 测试条件:5%sodium chloride liquid.5%氯化钠水溶液
SUBJECT 主题:SALT FOG TEST
盐雾测试
NO.REPORT 报告编号:AJTS0001/08
TEST APPLICATION测试申请
APPLY BRANCH 申请部门:
PRODUCT TYPE 产品型号:
COLOUR颜色:
TEST BRANCH 检测中心:QCCT
STANDARD标准 :
HOUR(H)时间/小时 24
48
72
APPEARANCEDEFEC T外观缺陷
PROTECTION RATING 保护评级
APPEARANCE RATING 外观评级
PERFORMANCE RATING性能评级 PROBLEM DESCRIPTOIN问题描述:
96
120
PHOTOS 图片:
124
168
SAMPLES样品数量: PC
MANUFACTURER供应商:
TEST REQUEST测试要求:
TEST TIME 测试时间(秒SEC.):
PROPOSTERA申请人:
APPROVE审阅 : DATE 日期:2007/12/21
APPEARANCE DEFECT外观缺陷 &保护等级关系POROTECT LEVEL REห้องสมุดไป่ตู้ATION
CHECKDE审阅:
盐雾试验报告

有限公司试 验 报 告Test Report报告编号/Report NO:20131010样品件号Specimen NO.样品材质Specimen Material 65Mn t=1.0试验设备Test Equipment 样品件名Specimen Name 螺栓、垫片、螺丝、车 型 VehicleModel 试验依据Confirm Specification GB/T18684-2002盐雾试验箱试验频率Test Frequency 开始/完成时间 Start/FinishTime 2014.09.02-2014.09.2试验区分Test Type■性能试验 / Assembly Test □材质试验 / Material Test 处理类别 Handle type■ 一般件 / general case □ 急件 / urgent case 样品数量Specimen Quantity3 pcs 试验项目Test Item 盐雾试验试验方法 /Test Methods判定基准/Requirements判定/ Judge □ 合格 / OK 4小时产生红锈4小时产生红锈试验前后样件图片 the Specimen pictures (before testing and after testing ):按国标GB/T GB/T18684-2002设定试验条件满足,将试样放入试验箱□ 无法判定 /Unable to determine报告结果,试验数据/Report the test result (testing data)□ 不合格 / NG4小时产生红锈试验初始温度湿度the temperature and relative humidityin initial test 常温试验完后温度湿度the temperature and relative humidity after testing常温样品委托单位Request Department工程部/Engineering Dept.样品是否保留Retain The Specimen Or Not□ 是 / YSE■否 / NO ■ 初期样品 Initial sample parts□ 进料检验Receiving inspection □ 工程监察Project □ 生产线问题Production line problem□ 其它Other 注意事项/Caution :送样来源Specimen source□ 客户要求 Customer RequestThis report is invalid without the common seal of the testing lab保管到无效 版本/修订 A/0 第1页,共1页批准Approved By审核Audited By编制Completed ByThis report is invalid without the signature or stamp of the complier ,auditor and approver1、本报告仅对送检样品负责;This report is only responsible for the specimen submitted for test2、无编制、审核、批准签章报告无效;3、报告无检验单位公章无效。
盐雾测试报告- 样张

试验时间
Test duration
月
日
时
Байду номын сангаас
至
月
日
时
共计
小时
1、盐水浓度:(5± 0.1)%所用盐为无水氯化钠,其碘化钠含量不得大于0.1% 总杂质含量不大于0.3%,盐溶液PH值6.5~7.2。 2、盐雾测试标准: GB 6458-86 3、等级判定标准: GB 5944-86 观察时间 项目 实验箱 温度 压力桶 温度 压缩空 气压力 喷雾量 盐溶液PH 试件外 观检查 要求 35± 1℃ 40± 1℃ 0.1~0.2 Kgf/cm2 1~2ml/ 80cm2/H 6.5~7.2 表面无锈 蚀现象 评判等级 Ratings 结 果 Test Result 测试员 Inspected By 审 核 Approved By 720 小 时 检查结果 观察时间 项目 实验箱 温度 压力桶 温度 压缩空 气压力 喷雾量 盐溶液PH 试件外 观检查 要求 35± 1℃ 40± 1℃ 0.1~0.2 Kgf/cm2 1~2ml/ 80cm2/H 6.5~7.2 表面无锈 蚀现象 检查结果
相关照片(Photos)
测试前照片 (Before Test) 360小时照片 (After 360 Hours)
720小时照片 (After 720 Hours)
720小时照片 (After 720 Hours)
盐雾试验测试报告 Salt Spray Test Report
客户
Custom er
报告编号
Report No.
版本号
Rev No.
A/1
样品名称
Component Name
样品材质
Componet Material
ASTM B117-07盐雾试验英文版

Designation:B 117–07Standard Practice forOperating Salt Spray (Fog)Apparatus 1This standard is issued under the fixed designation B 117;the number immediately following the designation indicates the year of original adoption or,in the case of revision,the year of last revision.A number in parentheses indicates the year of last reapproval.A superscript epsilon (e )indicates an editorial change since the last revision or reapproval.This standard has been approved for use by agencies of the Department of Defense.1.Scope1.1This practice covers the apparatus,procedure,and conditions required to create and maintain the salt spray (fog)test environment.Suitable apparatus which may be used is described in Appendix X1.1.2This practice does not prescribe the type of test speci-men or exposure periods to be used for a specific product,nor the interpretation to be given to the results.1.3The values stated in SI units are to be regarded as the standard.The values given in parentheses are for information only.1.4This standard does not purport to address all of the safety concerns,if any,associated with its use.It is the responsibility of the user of this standard to establish appro-priate safety and health practices and determine the applica-bility of regulatory limitations prior to use.2.Referenced Documents 2.1ASTM Standards:2B 368Test Method for Copper-Accelerated Acetic Acid-Salt Spray (Fog)Testing (CASS Test)D 609Practice for Preparation of Cold-Rolled Steel Panels for Testing Paint,Varnish,Conversion Coatings,and Related Coating ProductsD 1193Specification for Reagent WaterD 1654Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive EnvironmentsE 70Test Method for pH of Aqueous Solutions With the Glass ElectrodeE 691Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test MethodG 85Practice for Modified Salt Spray (Fog)Testing3.Significance and Use3.1This practice provides a controlled corrosive environ-ment which has been utilized to produce relative corrosion resistance information for specimens of metals and coated metals exposed in a given test chamber.3.2Prediction of performance in natural environments has seldom been correlated with salt spray results when used as stand alone data.3.2.1Correlation and extrapolation of corrosion perfor-mance based on exposure to the test environment provided by this practice are not always predictable.3.2.2Correlation and extrapolation should be considered only in cases where appropriate corroborating long-term atmo-spheric exposures have been conducted.3.3The reproducibility of results in the salt spray exposure is highly dependent on the type of specimens tested and the evaluation criteria selected,as well as the control of the operating variables.In any testing program,sufficient repli-cates should be included to establish the variability of the results.Variability has been observed when similar specimens are tested in different fog chambers even though the testing conditions are nominally similar and within the ranges speci-fied in this practice.4.Apparatus4.1The apparatus required for salt spray (fog)exposure consists of a fog chamber,a salt solution reservoir,a supply of suitably conditioned compressed air,one or more atomizing nozzles,specimen supports,provision for heating the chamber,and necessary means of control.The size and detailed con-struction of the apparatus are optional,provided the conditions obtained meet the requirements of this practice.4.2Drops of solution which accumulate on the ceiling or cover of the chamber shall not be permitted to fall on the specimens being exposed.1This practice is under the jurisdiction of ASTM Committee G01on Corrosion of Metals and is the direct responsibility of Subcommittee G01.05on Laboratory Corrosion Tests.Current edition approved March 1,2007.Published March 2007.Originally approved in st previous edition approved in 2003as B 117–03.2For referenced ASTM standards,visit the ASTM website,,or contact ASTM Customer Service at service@.For Annual Book of ASTM Standards volume information,refer to the standard’s Document Summary page on the ASTM website.Copyright ©ASTM International,100Barr Harbor Drive,PO Box C700,West Conshohocken,PA 19428-2959,United States.w ww .b zf xw .c o4.3Drops of solution which fall from the specimens shall not be returned to the solution reservoir for respraying.4.4Material of construction shall be such that it will not affect the corrosiveness of the fog.4.5All water used for this practice shall conform to Type IV water in Specification D 1193(except that for this practice limits for chlorides and sodium may be ignored).This does not apply to running tap water.All other water will be referred to as reagent grade.5.Test Specimens5.1The type and number of test specimens to be used,as well as the criteria for the evaluation of the test results,shall be defined in the specifications covering the material or product being exposed or shall be mutually agreed upon between the purchaser and the seller.6.Preparation of Test Specimens6.1Specimens shall be suitably cleaned.The cleaning method shall be optional depending on the nature of the surface and the contaminants.Care shall be taken that specimens are not recontaminated after cleaning by excessive or careless handling.6.2Specimens for the evaluation of paints and other organic coatings shall be prepared in accordance with applicable specification(s)for the material(s)being exposed,or as agreed upon between the purchaser and the supplier.Otherwise,the test specimens shall consist of steel meeting the requirements of Practice D 609and shall be cleaned and prepared for coating in accordance with the applicable procedure of Practice D 609.6.3Specimens coated with paints or nonmetallic coatings shall not be cleaned or handled excessively prior to test.6.4Whenever it is desired to determine the development of corrosion from an abraded area in the paint or organic coating,a scratch or scribed line shall be made through the coating with a sharp instrument so as to expose the underlying metal before testing.The conditions of making the scratch shall be as defined in Test Method D 1654,unless otherwise agreed upon between the purchaser and the seller.6.5Unless otherwise specified,the cut edges of plated,coated,or duplex materials and areas containing identification marks or in contact with the racks or supports shall be protected with a suitable coating stable under the conditions of the practice.N OTE 1—Should it be desirable to cut test specimens from parts or from preplated,painted,or otherwise coated steel sheet,the cut edges shall be protected by coating them with paint,wax,tape,or other effective media so that the development of a galvanic effect between such edges and the adjacent plated or otherwise coated metal surfaces,is prevented.7.Position of Specimens During Exposure7.1The position of the specimens in the salt spray chamber during the test shall be such that the following conditions are met:7.1.1Unless otherwise specified,the specimens shall be supported or suspended between 15and 30°from the vertical and preferably parallel to the principal direction of flow of fog through the chamber,based upon the dominant surface being tested.7.1.2The specimens shall not contact each other or any metallic material or any material capable of acting as a wick.7.1.3Each specimen shall be placed to permit unencum-bered exposure to the fog.7.1.4Salt solution from one specimen shall not drip on any other specimen.N OTE 2—Suitable materials for the construction or coating of racks and supports are glass,rubber,plastic,or suitably coated wood.Bare metal shall not be used.Specimens shall preferably be supported from the bottom or the side.Slotted wooden strips are suitable for the support of flat panels.Suspension from glass hooks or waxed string may be used as long as the specified position of the specimens is obtained,if necessary by means of secondary support at the bottom of the specimens.8.Salt Solution8.1The salt solution shall be prepared by dissolving 561parts by mass of sodium chloride in 95parts of water conforming to Type IV water in Specification D 1193(except that for this practice limits for chlorides and sodium may be ignored).Careful attention should be given to the chemical content of the salt.The salt used shall be sodium chloride with not more than 0.3%by mass of total impurities.Halides (Bromide,Fluoride,and Iodide)other than Chloride shall constitute less than 0.1%by mass of the salt content.Copper content shall be less than 0.3ppm by mass.Sodium chloride containing anti-caking agents shall not be used because such agents may act as corrosion inhibitors.See Table 1for a listing of these impurity restrictions.Upon agreement between the purchaser and the seller,analysis may be required and limitsTABLE 1Maximum Allowable Limits for Impurity Levels inSodium Chloride A ,B ,CImpurity DescriptionAllowable AmountTotal Impurities#0.3%Halides (Bromide,Fluoride and Iodide)excluding Chloride <0.1%Copper<0.3ppm Anti-caking AgentsnoneAA common formula used to calculate the amount of salt required by mass to achieve a 5%salt solution of a known mass of water is:0.0533Mass of Water 5Mass of NaCl requiredThe mass of water is 1g per 1mL.To calculate the mass of salt required in grams to mix 1L of a 5%salt solution,multiply 0.053by 1000g (35.27oz,the mass of 1L of water).This formula yields a result of 53g (1.87oz)of NaCl required for each liter of water to achieve a 5%salt solution by mass.The 0.053multiplier for the sodium chloride used above is derived by the following:1000g ~mass of a full L of water !divided by 0.95~water is only 95%of the total mixture by mass !yields 1053gThis 1053g is the total mass of the mixture of one L of water with a 5%sodium chloride concentration.1053g minus the original weight of the L of water,1000g,yields 53g for the weight of the sodium chloride.53g of total sodium chloride divided by the original 1000g of water yields a 0.053multiplier for the sodium chloride.As an example:to mix the equivalent of 200L (52.83gal)of 5%sodium chloride solution,mix 10.6kg (23.37lb)of sodium chloride into 200L (52.83gal)of water.200L of water weighs 200000g.200000g of water 30.053(sodium chloride multiplier)=10600g of sodium chloride,or 10.6kg.BIn order to ensure that the proper salt concentration was achieved when mixing the solution,it is recommended that the solution be checked with either a salimeter hydrometer or specific gravity hydrometer.When using a salimeter hydrometer,the measurement should be between 4and 6%at 25°C (77°F).When using a specific gravity hydrometer,the measurement should be between 1.0255and 1.0400at 25°C (77°F).CIf the purity of the salt used is >99.9%,then the limits for halides can be ignored.This is due to the fact that the halides cannot be $0.1%with a salt purity of >99.9%.If the salt used is of lower purity,then test forhalides.--````````,`,,,`,``,`,`,`````-`-`,,`,,`,`,,`---w ww .b zf xw .c oestablished for elements or compounds not specified in the chemical composition given above.8.2The pH of the salt solution shall be such that when atomized at 35°C (95°F)the collected solution will be in the pH range from 6.5to 7.2(Note 3).Before the solution is atomized it shall be free of suspended solids (Note 4).The pH measurement shall be made at 25°C (77°F)using a suitable glass pH-sensing electrode,reference electrode,and pH meter system in accordance with Test Method E 70.N OTE 3—Temperature affects the pH of a salt solution prepared from water saturated with carbon dioxide at room temperature and pH adjust-ment may be made by the following three methods:(1)When the pH of a salt solution is adjusted at room temperature,and atomized at 35°C (95°F),the pH of the collected solution will be higher than the original solution due to the loss of carbon dioxide at the higher temperature.When the pH of the salt solution is adjusted at room temperature,it is therefore necessary to adjust it below 6.5so the collected solution after atomizing at 35°C (95°F)will meet the pH limits of 6.5to 7.2.Take about a 50-mL sample of the salt solution as prepared at room temperature,boil gently for 30s,cool,and determine the pH.When the pH of the salt solution is adjusted to 6.5to 7.2by this procedure,the pH of the atomized and collected solution at 35°C (95°F)will come within this range.(2)Heating the salt solution to boiling and cooling to 35°C (95°F)and maintaining it at 35°C (95°F)for approximately 48h before adjusting the pH produces a solution the pH of which does not materially change when atomized at 35°C (95°F).(3)Heating the water from which the salt solution is prepared to 35°C (95°F)or above,to expel carbon dioxide,and adjusting the pH of the salt solution within the limits of 6.5to 7.2produces a solution the pH of which does not materially change when atomized at 35°C (95°F).N OTE 4—The freshly prepared salt solution may be filtered or decanted before it is placed in the reservoir,or the end of the tube leading from the solution to the atomizer may be covered with a double layer of cheesecloth to prevent plugging of the nozzle.N OTE 5—The pH can be adjusted by additions of dilute ACS reagent grade hydrochloric acid or sodium hydroxide solutions.9.Air Supply9.1The compressed air supply to the Air Saturator Tower shall be free of grease,oil,and dirt before use by passing through well-maintained filters.(Note 6)This air should be maintained at a sufficient pressure at the base of the Air Saturator Tower to meet the suggested pressures of Table 2at the top of the Air Saturator Tower.N OTE 6—The air supply may be freed from oil and dirt by passing it through a suitable oil/water extractor (that is commercially available)to stop any oil from reaching the Air Saturator Tower.Many oil/water extractors have an expiration indicator,proper preventive maintenance intervals should take these into account.9.2The compressed air supply to the atomizer nozzle or nozzles shall be conditioned by introducing it into the bottomof a tower fillwed with water.A common method of introduc-ing the air is through an air dispersion device (X1.4.1).The level of the water must be maintained automatically to ensure adequate humidification.It is common practice to maintain the temperature in this tower between 46and 49°C (114–121°F)to offset the cooling effect of expansion to atmospheric pressure during the atomization process.Table 2shows the temperature,at different pressures,that are commonly used to offset the cooling effect of expansion to atmospheric pressure.9.3Careful attention should be given to the relationship of tower temperature to pressure since this relationship can have a direct impact to maintaining proper collection rates (Note 7).It is preferable to saturate the air at temperatures well above the chamber temperature as insurance of a wet fog as listed in Table 2.N OTE 7—If the tower is run outside of these suggested temperature and pressure ranges to acheive proper collection rates as described in 10.2of this practice,other means of verifying the proper corrosion rate in the chamber should be investigated,such as the use of control specimens (panels of known performance in the test conducted).It is preferred that control panels be provided that bracket the expected test specimen performance.The controls allow for the normalization of test conditions during repeated running of the test and will also allow comparisons of test results from different repeats of the same test.(Refer to Appendix X3,Evaluation of Corrosive Conditions,for mass loss procedures).10.Conditions in the Salt Spray Chamber10.1Temperature —The exposure zone of the salt spray chamber shall be maintained at 3562°C (9563°F).Each set point and its tolerance represents an operational control point for equilibrium conditions at a single location in the cabinet which may not necessarily represent the uniformity of condi-tions throughout the cabinet.The temperature within the exposure zone of the closed cabinet shall be recorded (Note 8)at least once daily (except on Saturdays,Sundays,and holidays when the salt spray test is not interrupted for exposing,rearranging,or removing test specimens or to check and replenish the solution in the reservoir)N OTE 8—A suitable method to record the temperature is by a continu-ous recording device or by a thermometer which can be read from outside the closed cabinet.The recorded temperature must be obtained with the salt spray chamber closed to avoid a false low reading because of wet-bulb effect when the chamber is open.10.2Atomization and Quantity of Fog —Place at least two clean fog collectors per atomizer tower within the exposure zone so that no drops of solution will be collected from the test specimens or any other source.Position the collectors in the proximity of the test specimens,one nearest to any nozzle and the other farthest from all nozzles.A typical arrangement is shown in Fig.1.The fog shall be such that for each 80cm 2(12.4in.2)of horizontal collecting area,there will be collected from 1.0to 2.0mL of solution per hour based on an average run of at least 16h (Note 9).The sodium chloride concentration of the collected solution shall be 561mass %(Notes 9-11).The pH of the collected solution shall be 6.5to 7.2.The pH measurement shall be made as described in 8.2(Note 3).N OTE 9—Suitable collecting devices are glass or plastic funnels withTABLE 2Suggested Temperature and Pressure guideline for the top of the Air Saturator Tower for the operation of a test at 35°C(95°F)Air Pressure,kPaTemperature,°CAir Pressure,PSITemperature,°F83461211496471411711048161191244918121--````````,`,,,`,``,`,`,`````-`-`,,`,,`,`,,`---w ww .b zf xw .c othe stems inserted through stoppers into graduated cylinders,or crystal-lizing dishes.Funnels and dishes with a diameter of 10cm (3.94in.)have an area of about 80cm 2(12.4in.2).N OTE 10—A solution having a specific gravity of 1.0255to 1.0400at 25°C (77°F)will meet the concentration requirement.The sodium chloride concentration may also be determined using a suitable salinity meter (for example,utilizing a sodium ion-selective glass electrode)or colorimetrically as follows.Dilute 5mL of the collected solution to 100mL with distilled water and mix thoroughly;pipet a 10-mL aliquot into an evaporating dish or casserole;add 40mL of distilled water and 1mL of 1%potassium chromate solution (chloride-free)and titrate with 0.1N silver nitrate solution to the first appearance of a permanent red coloration.A solution that requires between 3.4and 5.1mL of 0.1N silver nitrate solution will meet the concentration requirements.N OTE 11—Salt solutions from 2to 6%will give the same results,though for uniformity the limits are set at 4to 6%.10.3The nozzle or nozzles shall be so directed or baffled that none of the spray can impinge directly on the test specimens.11.Continuity of Exposure11.1Unless otherwise specified in the specifications cover-ing the material or product being tested,the test shall be continuous for the duration of the entire test period.Continu-ous operation implies that the chamber be closed and the spray operating continuously except for the short daily interruptions necessary to inspect,rearrange,or remove test specimens,to check and replenish the solution in the reservoir,and to make necessary recordings as described in Section 10.Operations shall be so scheduled that these interruptions are held to a minimum.12.Period of Exposure12.1The period of exposure shall be as designated by the specifications covering the material or product being tested or as mutually agreed upon between the purchaser and the seller.N OTE 12—Recommended exposure periods are to be as agreed upon between the purchaser and the seller,but exposure periods of multiples of 24h are suggested.13.Cleaning of Tested Specimens13.1Unless otherwise specified in the specifications cover-ing the material or product being tested,specimens shall be treated as follows at the end of the test:13.1.1The specimens shall be carefully removed.13.2Specimens may be gently washed or dipped in clean running water not warmer than 38°C (100°F)to remove salt deposits from their surface,and then immediately dried.14.Evaluation of Results14.1A careful and immediate examination shall be made as required by the specifications covering the material or product being tested or by agreement between the purchaser and the seller.15.Records and Reports15.1The following information shall be recorded,unless otherwise prescribed in the specifications covering the material or product being tested:15.1.1Type of salt and water used in preparing the salt solution,15.1.2All readings of temperature within the exposure zone of the chamber,15.1.3Daily records of data obtained from each fog-collecting device including the following:15.1.3.1V olume of salt solution collected in millilitres per hour per 80cm 2(12.4in.2),15.1.3.2Concentration or specific gravity at 35°C (95°F)of solution collected,and15.1.3.3pH of collected solution.N OTE —This figure shows a typical fog collector arrangement for a single atomizer tower cabinet.The same fog collector arrangement is also applicable for multiple atomizer tower and horizontal (“T”type)atomizer tower cabinet constructions as well.FIG.1Arrangement of FogCollectors--````````,`,,,`,``,`,`,`````-`-`,,`,,`,`,,`---w ww .b zf xw .c o15.2Type of specimen and its dimensions,or number or description of part,15.3Method of cleaning specimens before and after testing,15.4Method of supporting or suspending article in the salt spray chamber,15.5Description of protection used as required in 6.5,15.6Exposure period,15.7Interruptions in exposure,cause,and length of time,and15.8Results of all inspections.N OTE 13—If any of the atomized salt solution which has not contacted the test specimens is returned to the reservoir,it is advisable to record the concentration or specific gravity of this solution also.16.Keywords16.1controlled corrosive environment;corrosive condi-tions;determining mass loss;salt spray (fog)exposureAPPENDIXES(Nonmandatory Information)X1.CONSTRUCTION OF APPARATUSX1.1CabinetsX1.1.1Standard salt spray cabinets are available from several suppliers,but certain pertinent accessories are required before they will function according to this practice and provide consistent control for duplication of results.X1.1.2The salt spray cabinet consists of the basic chamber,an air-saturator tower,a salt solution reservoir,atomizing nozzles,specimen supports,provisions for heating the cham-ber,and suitable controls for maintaining the desired tempera-ture.X1.1.3Accessories such as a suitable adjustable baffle or central fog tower,automatic level control for the salt reservoir,and automatic level control for the air-saturator tower are pertinent parts of the apparatus.X1.1.4The size and shape of the cabinet shall be such that the atomization and quantity of collected solution is within the limits of this practice.X1.1.5The chamber shall be made of suitably inert mate-rials such as plastic,glass,or stone,or constructed of metal and lined with impervious plastics,rubber,or epoxy-type materials or equivalent.X1.1.6All piping that contacts the salt solution or spray should be of inert materials such as plastic.Vent piping should be of sufficient size so that a minimum of back pressure exists and should be installed so that no solution is trapped.The exposed end of the vent pipe should be shielded from extreme air currents that may cause fluctuation of pressure or vacuum in the cabinet.X1.2Temperature ControlX1.2.1The maintenance of temperature within the salt chamber can be accomplished by several methods.It is generally desirable to control the temperature of the surround-ings of the salt spray chamber and to maintain it as stable as possible.This may be accomplished by placing the apparatus in a constant-temperature room,but may also be achieved by surrounding the basic chamber of a jacket containing water or air at a controlled temperature.X1.2.2The use of immersion heaters in an internal salt solution reservoir or within the chamber is detrimental whereheat losses are appreciable because of solution evaporation andradiant heat on the specimens.X1.3Spray NozzlesX1.3.1Satisfactory nozzles may be made of hard rubber,plastic,or other inert materials.The most commonly used type is made of plastic.Nozzles calibrated for air consumption and solution-atomized are available.The operating characteristics of a typical nozzle are given in Table X1.1.X1.3.2It can readily be seen that air consumption is relatively stable at the pressures normally used,but a marked reduction in solution sprayed occurs if the level of the solution is allowed to drop appreciably during the test.Thus,the level of the solution in the salt reservoir must be maintained automatically to ensure uniform fog delivery during the test.3X1.3.3If the nozzle selected does not atomize the salt solution into uniform droplets,it will be necessary to direct the spray at a baffle or wall to pick up the larger drops and prevent them from impinging on the test specimens.Pending a com-plete understanding of air-pressure effects,and so forth,it is important that the nozzle selected shall produce the desired3A suitable device for maintaining the level of liquid in either the saturator tower or reservoir of test solution may be designed by a local engineering group,or it may be purchased from manufacturers of test cabinets as an accessory.TABLE X1.1Operating Characteristics of Typical Spray NozzleSiphon Height ,cm Air Flow,dm 3/min Solution Consumption,cm 3/hAir Pressure,kPa Air Pressure,kPa34691031383469103138101926.531.5362100384045845256201926.531.536636276037204320301926.531.5360138030003710401926.631.536078021242904Siphon Height,in.Air Flow,L/minSolutionConsumption,mL/h Air Pressure,psi Air Pressure,psi 5101520510152041926.531.536210038404584525681926.531.536636276037204320121926.531.5360138030003710161926.631.53678021242904--````````,`,,,`,``,`,`,`````-`-`,,`,,`,`,,`---w ww .b zf xw .c ocondition when operated at the air pressure selected.Nozzles are not necessarily located at one end,but may be placed in the center and can also be directed vertically up through a suitable tower.X1.4Air for AtomizationX1.4.1The air used for atomization must be free of grease,oil,and dirt before use by passing through well-maintained filters.Room air may be compressed,heated,humidified,and washed in a water-sealed rotary pump if the temperature of the water is suitably controlled.Otherwise cleaned air may be introduced into the bottom of a tower filled with water through a porous stone or multiple nozzles.The level of the water must be maintained automatically to ensure adequate humidification.A chamber operated in accordance with this method and Appendix X1will have a relative humidity between 95and 98%.Since salt solutions from 2to 6%will give the same results (though for uniformity the limits are set at 4to 6%),it is preferable to saturate the air at temperatures well above the chamber temperature as insurance of a wet fog.Table X1.2shows the temperatures,at different pressures,that are required to offset the cooling effect of expansion to atmospheric pressure.X1.4.2Experience has shown that most uniform spray chamber atmospheres are obtained by increasing the atomizing air temperature sufficiently to offset heat losses,except those that can be replaced otherwise at very low-temperature gradi-ents.X1.5Types of ConstructionX1.5.1A modern laboratory cabinet is shown in Fig.X1.1.Walk-in chambers are usually constructed with a sloping ceiling.Suitably located and directed spray nozzles avoid ceiling accumulation and drip.Nozzles may be located at the ceiling,or 0.91m (3ft)from the floor directed upward at 30to 60°over a passageway.The number of nozzles depends on type and capacity and is related to the area of the test space.An 11to 19L (3to 5-gal)reservoir is required within the chamber,with the level controlled.The major features of a walk-in type cabinet,which differs significantly from the laboratory type,are illustrated in Fig.X1.2.Construction of a plastic nozzle,such as is furnished by several suppliers,is shown in Fig.X1.3.TABLE X1.2Temperature and Pressure Requirements forOperation of Test at 95°FAir Pressure,kPa 8396110124Temperature,°C46474849Air Pressure,psi 12141618Temperature,°F114117119121--````````,`,,,`,``,`,`,`````-`-`,,`,,`,`,,`---w ww .b zf xw .c o。
盐雾试验表格Salt Spray test report-150714 Form - 空白
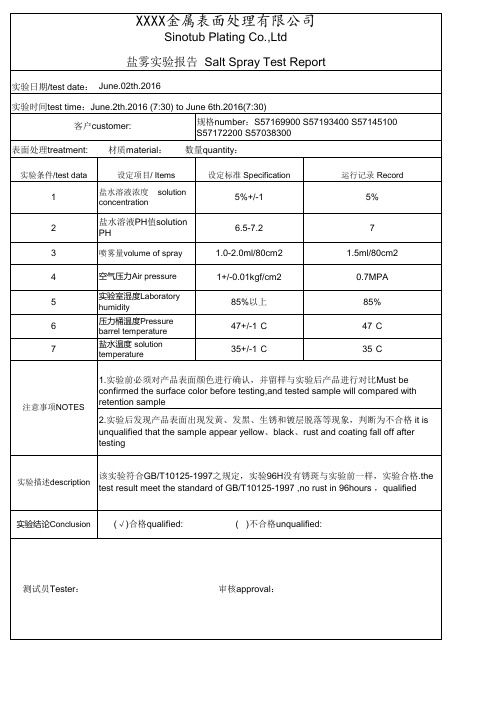
Sinotub Plating Co.,Ltd 盐雾实验报告 Salt Spray Test Report
实验日期/test date: June.02th.2016 实验时间test time:June.2th.2016 (7:30) to June 6th.2016(7:30) 客户customer: 表面处理treatment:
(√)合格qualified:
( )不合格unqualified:
测试员Tester:
审核approval:
1 2 3 Hale Waihona Puke 5 6 7solution
5%+/-1 6.5-7.2 1.0-2.0ml/80cm2 1+/-0.01kgf/cm2 85%以上 47+/-1° C 35+/-1° C
5% 7 1.5ml/80cm2 0.7MPA 85% 47° C 35° C
盐水溶液PH值solution PH
喷雾量volume of spray 空气压力Air pressure 实验室湿度Laboratory humidity 压力桶温度Pressure barrel temperature 盐水温度 solution temperature
注意事项NOTES
1.实验前必须对产品表面颜色进行确认,并留样与实验后产品进行对比Must be confirmed the surface color before testing,and tested sample will compared with retention sample 2.实验后发现产品表面出现发黄、发黑、生锈和镀层脱落等现象,判断为不合格 it is unqualified that the sample appear yellow、black、rust and coating fall off after testing
盐雾试验测试报告

J5\J6
13.23 15.69 14.12 13.32 14.15 12.32 12.36 14.15 15.33 15.63
J7\2 12.41 13.33 15.32 13.26 12.63 14.02 14.15
平均值 Average 13.67 15.12 13.08 12.75 13.19 14.25 12.99 13.54 14.92 14.88
判定 Result
OK OK OK OK OK OK OK OK OK OK
判定Judgement■合格PASS □不合格FAIL
审核Approveled:
制表Prepared:
7
oxidize bad
phenomenon 8
9
10
J1\J2
15.02 14.12 12.63 13.23 12.63 14.02 12.23 15.02 15.02 16.52
J3\J4
12.32 15.33 13.26 12.02 12.63 15.32 14.12 12.36 15.32 13.23
1.用20倍显微镜观察产品表面无腐蚀现象。
判定标准 1. No corrosion on the surface of the product was observed by 20 times
Requiremen microscope.
t
2.要求测试前与测试后的接触阻抗△≤10mΩ,结果≤30mΩ
Resistance is less than 10mΩ,result≤30mΩ
使用设备 Equipment
盐雾试验箱 Salt spray
测试日期 test date
序号
测试前结果(单位:毫欧)test record
盐雾测试 英文版

Salt spray testingSCOPE: this standard prescribles the conditions required in salt spray testing for specification purpose. It dose not prescribe the type of the specimen or sxposure periods to be used for a specific product, nor the interpretation to be given to the results.APPARATUS: 2.1 the apparatus required for salt spray testing consists of a fog chamber, a salt solution reservoir, a supply of suitably conditioned compressed air, one or more atomizing nozzles, specimen supports, provision for heating the chamber, and necessary means of control. The size and detailed the conditions obtained meet the requirements of this method. 2.2. drop of solution which accumulate on the celling or cover of the chamber shall not be permitted to fall on the specimens being tested. 2.3. drops of solution which fall from the specimens shall not be returned to the solution reservoir for respraying. 2.4. materials of construction shall be such that they shall not affect the correctiveness of the fog, nor be themselves corroded by the fog.TEST SPECIMENS: the type and number of test specimens to be used, as well as the criteria for the evaluation of the test results, shall be defined in the specifications covering the material or product being tested of shall be natually agreed upon by the purchaser and the supplier.PREPARATION OF TEST SPECIMENS 4.1. matallic and metallic-coated specimens shall be suitably cleared. The clearing method shall be optional depending on the nature of the surface and the contaminants, except that it shall not include the use of abrasives other than a parts of pure maggssium oxide nor of soivents which are cerretive or will deposit either corrosive or protective films. The ues of a nitric acid solution for the chemical cleaning, or passivation, of stainless steel specimens is permissible when agreed upon by the purchaser and the supplier. Care shall be taken that specimens are not recontaminated after caleaning by excessivs or careless handling. 4.2. specimens for evalustion of paints and other organic coasting shall be prepared according to applicable specification for the material being tested, or as agreed upon by the purchaser and aupplier. Otherwise the specimens shall consist of steel meetion the requirements of the methods for preparation of steel panels for testion paint, varnish, lacquer, and relate products and shall be cleaned and prepared for coation according to procedure A OF ASTM D609. 4.3. specimens coated with paints of nonmetallic coations shall not be cleaned of handled excessively prior to test. Whenever it is desired to determine the development of corrision from an abraded area in the paint of organic coation, a scratch of scribed line shall be made through the coation with a sharp instrument so as to expose the underlying metal before testing, the conditions of making the scratch shall be agreed upon between the purchaser and supplier. 4.5. unless otherwise specified, the cut edges of plated, coated, or duplex materials and areas containing identification marks or in contact with the racks of supports, shall be protected with a suitable coating stable under the conditions of the test, such as ceresin wax.POSITION OF SPECIMENS DURING TEST the position of the specimens in the salt spray chamber during the test shall be such that the following conditions are met. 5.1.unless otherwise specified, the specimens shall be supported or suspended between 15 and 30 degrees from the vertical and preferably parallel to the principle direction of horizontal flow of fog through the chamber, bassed upon the dominant surface being tested. 5.2. each specimen shall not contact each other or any metallic material of any material capable of acting as a wick. .5.3. each specimens shall be so placed as to permit free setting of fog on all specimens.TEST SOLUSION the salt solution shall be prepared by dissolving 5g of salt per every95ml of distilled water of water containing not more than 200 ppm of total solids. The salt used shall be sodium chloride substantially free of nickel and copper and containing on the dry basis not more than 0.1 percent of sodium iodide and not more than 0.3 percent of total impurities.CONDITIONS IN THE TEST CHAMBER 8.1. TEMPERARTURE the exposure zone of the salt chamber shall be maintained at 35 plus 1.1 or minus 1.7℃. the temperature within the exposure zone of the clossed cabinet shall be recorded at least twice a day at least 7 hours apart. 8.1. ATOMIZATION AND QUANTITY OF FOG at least two clean fog collectors shall be so placed within the exposure zone that no drops of solution from the test specimens of any other source shall be collected, the collectors shall be placed in the proximity of the test specimens, one nearest to any nozzle and the other farthest from all nozzles. The fog shall be such that for each 80 C㎡horizontal collection area there will be collected in each collector from 1.0 to 2.0 mL of solution per hour based on an average run of at least 16 hours. The sodiun chloride concentration of the collected solution shall be 5±1 percen mass. The pH of the collected solution shall be 6.5 to 7.2. the pH measurement shall be made electrometrically of colorimetrically using Bromthymol blue as the indicator. 8.3. the nozzle of nozzles shall be so directed or baffled that none of the spray can impinge directly on the test specimens. 8.4.dilution and evsporation of condensate should be avoided.CONTINUIY OF TEST unless otherwise specified in the specifications covering the material of product being tested, the test shall be continuous for the duration of the entire test period. Continuous operation implies that the chamber be clossed and the spray operation continuously except for the short daily interruptions necessary recordings as described in section8. operations shall be so scheduled that these interruptions are held to a minimum. PERIOD OF TEST. The period of test shall be as decignated by the specification covering the material of product being tested, or as mutually agreed by the purchaser and supplier. CLEANING OF TESTED SPECIMENS unless otherwise specified in the specification covering the material of product being tested, specimens shall be treated aws follows at the end of the test. 11.1 the specimens shall be carefully removed from the chamber. 11.2 specimens shall be gently rinsed in clean running warm water to remove salt deposits fromtheir surface, and then immediately dried. Drying shall be accoraplished with a stream of clean, compredded air at a gage pressure of 240 to 25 kPa.CVALUATION OF RESULTS a carefully and immediate examinatin shall be made for the extent of corrosion of the dry test specimens of for other failure as required by the specification covering the material or product being tested or by agreement between the purchaser and supplier.RECORDS AND REPORTS the following information shall be recorded, unless otherwise prescribed in the specification covering the material of product being tested. 13.1 type of salt and water used in preparing the salt solution. 13.2 all readings of temperature within the exposure zone of the chamber. 13.3 daily records of data obtained from each fog collecting device, including the following. 13.4 volume of salt solution collected in millilitres per hour per 80 C㎡13.5 concentration or specific gavity at 35℃of colution collected. 13.6 pH of collected solution. 13.7 type of specimen and dimension thereof or part number of description of part. 13.8. method of cleaning specimens before and ater testing. 13.9 method of supporting of suspending article in the salt apray chamber. 13.10. description of protection used as required in section 4. 13.11 exposure period. 13.12 inerruptions in test, cause and length of time. 13.13 results of all inspections.。
