Hydration of α-pinene homogenous catalyzed by acidic polyether-modified ammonium salt ionic liquid

Res Chem Intermed
DOI10.1007/s11164-013-1355-1
Hydration of a-pinene homogenous catalyzed by acidic polyether-modi?ed ammonium salt ionic liquid
in‘‘microreactor’’
Lu Li?Yue Liu?Shi-Tao Yu?Shi-Wei Liu?
Cong-Xia Xie?Fu-Sheng Liu
Received:17March2013/Accepted:29July2013
óSpringer Science+Business Media Dordrecht2013
Abstract Water-soluble ionic liquids(ILs)with the nonionic polyether modi?ed ammonium(Ac18n)as cation and[HSO4]-,[H2PO3]-,[PTSA]-,or[BF4]-as anion were synthesized and the critical solution temperature(CST)of these ILs in a-pinene were studied.The results showed that the new ILs([Ac1820]?[HSO4]-and [Ac1820]?[H2PO4]-)were of CST in a-pinene at80°C.Then,the new ILs was applied in hydration of a-pinene as catalysts.During the process of reaction,catalysts would carry water molecules to phase of a-pinene and therefore to form the micro-group of water-in-oil.The micro-group is regarded as a micro-reactor for its acidic group,in which the catalytic reaction would have taken place homogeneously when the reaction temperature(T)is higher than CST.When the reaction completed(T\CST),the catalyst would precipitate out from the product,and be recycled ef?ciently.
Keywords HydrationáPineneáCSTáMicro-reactoráIonic liquids Introduction
a-Terpineol,an important monoterpenic monocyclic alcohol,has several applica-tions in the perfume and pharmaceutical industries.It is obtained industrially through Electronic supplementary material The online version of this article(doi:10.1007/s11164-013-1355-1) contains supplementary material,which is available to authorized users.
L.Li(&)áY.LiuáS.-T.YuáS.-W.LiuáF.-S.Liu
College of Chemical Engineering,Qingdao University of Science and Technology,
53Zhengzhou Road,Qingdao266042,People’s Republic of China
e-mail:zhanglilu@https://www.360docs.net/doc/022309171.html,
C.-X.Xie
Key Laboratory of Eco-Chemical Engineering,Ministry of Education,
College of Chemistry and Molecular Engineering,Qingdao University of Science and Technology,
53Zhengzhou Road,Qingdao266042,People’s Republic of China
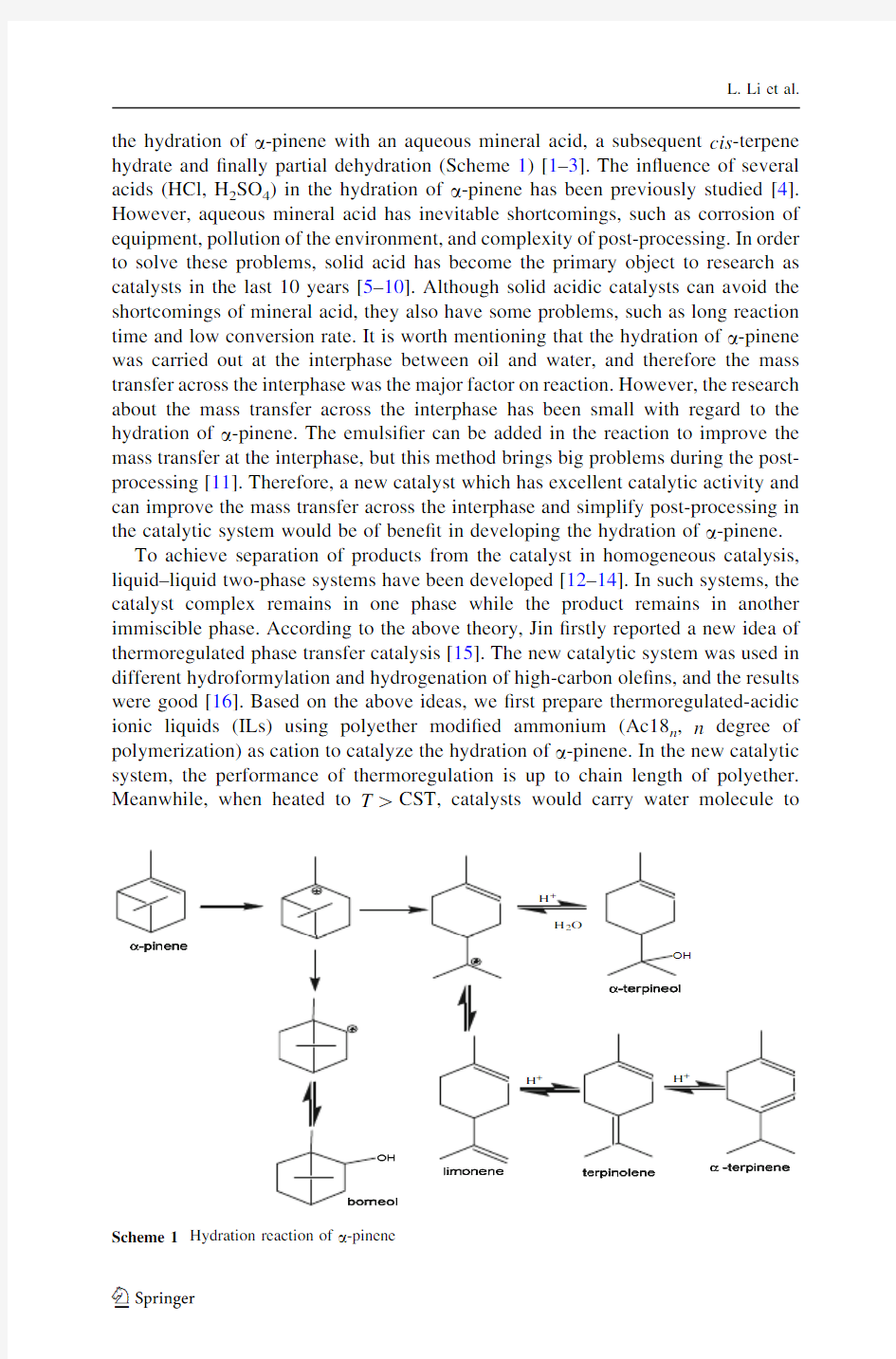
L.Li et al. the hydration of a-pinene with an aqueous mineral acid,a subsequent cis-terpene hydrate and?nally partial dehydration(Scheme1)[1–3].The in?uence of several acids(HCl,H2SO4)in the hydration of a-pinene has been previously studied[4]. However,aqueous mineral acid has inevitable shortcomings,such as corrosion of equipment,pollution of the environment,and complexity of post-processing.In order to solve these problems,solid acid has become the primary object to research as catalysts in the last10years[5–10].Although solid acidic catalysts can avoid the shortcomings of mineral acid,they also have some problems,such as long reaction time and low conversion rate.It is worth mentioning that the hydration of a-pinene was carried out at the interphase between oil and water,and therefore the mass transfer across the interphase was the major factor on reaction.However,the research about the mass transfer across the interphase has been small with regard to the hydration of a-pinene.The emulsi?er can be added in the reaction to improve the mass transfer at the interphase,but this method brings big problems during the post-processing[11].Therefore,a new catalyst which has excellent catalytic activity and can improve the mass transfer across the interphase and simplify post-processing in the catalytic system would be of bene?t in developing the hydration of a-pinene.
To achieve separation of products from the catalyst in homogeneous catalysis, liquid–liquid two-phase systems have been developed[12–14].In such systems,the catalyst complex remains in one phase while the product remains in another immiscible phase.According to the above theory,Jin?rstly reported a new idea of thermoregulated phase transfer catalysis[15].The new catalytic system was used in different hydroformylation and hydrogenation of high-carbon ole?ns,and the results were good[16].Based on the above ideas,we?rst prepare thermoregulated-acidic ionic liquids(ILs)using polyether modi?ed ammonium(Ac18n,n degree of polymerization)as cation to catalyze the hydration of a-pinene.In the new catalytic system,the performance of thermoregulation is up to chain length of polyether. Meanwhile,when heated to T[CST,catalysts would carry water molecule to
OH
H+H+
Scheme1Hydration reaction of a-pinene
Modi?ed ammonium salt ionic liquid
phase of a-pinene and at the same time the micro group of water-in-oil was formed in the phase of a-pinene.Then,each micro-group of water-in-oil was of the acidic group,and therefore the micro-group is regarded as a micro-reactor.In each micro-reactor,the contact of water and a-pinene strengthened,so the reaction could be easily carried out.After the reaction(T\CST),the catalyst precipitates from the organic phase,and can be easily separated from the product and recycled ef?ciently. In this paper,the catalytic mechanism and the relevant factors that affect the reaction were carefully discussed.The catalyst has the advantages of aqueous and solid acid,it is a good start to develop the hydration of a-pinene.A preliminary report of this work has been presented elsewhere[17].
Experimental
Materials
All materials,such as a-pinene,anhydrous magnesium sulfate,octadecylamine polyoxyethylene(1,812,1,815,1,820,1,830),1,3-propanesulfonic acid lactone(1,3-PS),sulfuric acid,phosphoric acid,p-toluenesulfonic acid(PTSA),tetra?uorobo-rate,were all purchased from Aldrich,and all materials were directly used after drying without further puri?cation.
Reaction experiments
The synthetic reaction of[Ac18n]?[HSO4]-was performed in a100-cm3three-necked glass reactor with a refrigerant and a thermocouple.The reactor was submerged in a thermostatic bath with silicone oil and magnetic stirring.In batch experiments,a certain amount of Ac18n and deionized water was?rst added.After heating to the desired temperature,the equal-mole amount of sulfuric acid was added,and then heated in an oil-bath for2h.The water of the mixture was then removed under vacuum(5–10mmHg)at90°C,then a light yellow solid [Ac18n]?[HSO4]-was obtained,and the product was analysed with FT-IR,1H NMR,and13C NMR.[Ac18n]?[H2PO3]-,[Ac18n]?[PTSA]-,and[Ac18n]?[BF4]-were prepared in the same way(Scheme2).
The tests of CST were performed in a200-cm3three-necked glass reactor with a refrigerant and a thermocouple.The reactor was submerged in a thermostatic bath with silicone oil and magnetic stirring.The ratio of a-pinene,ILs,and water was n(a-pinene):n(ILs):n(water)=1:0.05:5.The a-pinene,ILs,and water were added to the reactor and heated.When the solvent system became homogeneous phase,the temperature was CST.
The catalytic tests were performed in a200-cm3three-necked glass reactor with a refrigerant and a thermocouple.The reactor was submerged in a thermostatic bath with silicone oil and magnetic stirring.In batch experiments,0.06mol of a-pinene, and5.4mL of water were?rst added.After heating to the desired temperature,the catalyst was added.Aliquots were extracted with a micropipette at?xed times and immediately analyzed with a GC17Shimadzu gas chromatographer equipped with
L.Li et al.
an FID and a capillary DB1column(60m in length).The reaction products were identi?ed by comparing the retention times of terpene standards or using their Kovats index with con?rmation by mass spectroscopy.The ILs can be directly used in the second cyclic utilization(Scheme1).
Results and discussion
To realize the idea mentioned above,we designed new acidic ILs using Ac18n as cation that could be dissolved in a-pinene at high temperature and could not be dissolved in a-pinene at low temperature.The dissolving behavior of new catalysts in a-pinene and water was investigated at different temperatures(Table1).From Table1,it can be seen that ILs with different acidic groups as anion were of different solution temperatures in a-pinene.The ILs with[H2PO4]and[HSO4]as anion could be dissolved readily in a-pinene when the temperature was more than 80°C,and could not be dissolved in a-pinene and formed two phases when the temperature was less than80°https://www.360docs.net/doc/022309171.html,ing[PTSA]as anion,the ILs could be dissolved in a-pinene when the temperature was more than100°C,and be partly dissolved in a-pinene when the temperature was less than100°C.However,using[BF4]as anion,the ILs could not be dissolved in a-pinene when the temperature was more than140°C.The results showed that the new ILs([Ac1820]?[HSO4]-and [Ac1820]?[H2PO4]-)were of CST in a-pinene at80°C.The dissolving behavior of these ILs in a-pinene and water was investigated using[Ac1820]?[HSO4]-as an example.It suggested that the thermal motion of molecules between [Ac1820]?[HSO4]-and a-pinene would strengthen with a rise of temperature,so that the system became uniform phase.When the temperature decreased,thermal motion of molecules between[Ac1820]?[HSO4]-and pinene would weaken.At the impact of gravity,[Ac1820]?[HSO4]-would move down,and the intermiscibility between[Ac1820]?[HSO4]-and a-pinene reduced,so the system become two phases.At the same time,the effect of degree of polymerization(n)of [Ac18n]?[HSO4]-and[Ac18n]?[H2PO4]-on the dissolving behavior was also studied(Table2).From Table2,it can be seen that n greatly affected the dissolving behavior between ILs and pinene.When n is less than20,with the increasing of temperature,[Ac18n]?[HSO4]–and[Ac18n]?[H2PO4]-cannot be dissolved in a-pinene,and the same phenomenon can be observed when n is30.Only when n is20,[Ac1820]?[HSO4]-and[Ac1820]?[H2PO4]-can be dissolved in a-pinene at a temperature more than80°C.From the above result,80°C is the CST of
Modi?ed ammonium salt ionic liquid
[Ac1820]?[HSO4]-and[Ac1820]?[H2PO4]-in a-pinene.For the hydration of a-pinene,80°C was a suitable temperature[1].Next,the catalytic activity of [Ac1820]?[HSO4]-and[Ac1820]?[H2PO4]-on the hydration of a-pinene was researched.
It can be seen from Table3that the[Ac1820]?[HSO4]-is of good catalytic activity,and its selectivity to a-terpineol is more than aqueous acid.That result must be relative to the thermoregulated performance of[Ac1820]?[HSO4]-.According to the dissolving mechanism,the phenomenon of the reaction can be explained as Fig.1.At room temperature,the hydrogen bond between catalyst and water makes the catalyst disperse uniformly in water.On heating to the temperature,the hydrogen bond between the catalyst and water would weaken and the catalyst would dissolve in a-pinene.In the process of dissolving,the catalysts would carry water molecular to the phase of a-pinene and at the same time the micro-group of water-in-oil was formed in the phase of a-pinene[19].Then,every micro-group was regarded as a micro-reactor[20].In every micro-reaction,a-pinene could easily be catalyzed to the aimed production.When the reaction completed,catalysts precipitate out from the compound of the product,and could be easily separated from the product and recycled ef?ciently.For the two ILs,[Ac1820]?[HSO4]-has the better catalytic activity and selectivity to a-terpineol,which can be explained from their acidity.FT-py spectra is an effective tool to verify the type of acid,as Table1The dissolving behavior of different ILs in a-pinene at different temperature
T(°C)[Ac1820]?[H2PO4]-[Ac1820]?[HSO4]-[Ac1820]?[PTSA]-[Ac1820]?[BF4]-
40i i ps ps
60i i ps ps
80s s ps ps
100s s s ps
120s s–ps
140–––ps
s Solubilization,ps part solubilization,i insolubilization;n(a-pinene):n(ILs):n(water)1:0.05:5
Table2The dissolving behavior of different degree of polymerization(n)ionic liquids in a-pinene at different temperatures
T(°C)[Ac18n]?[H2PO4]-[Ac18n]?[HSO4]-
1215203012152030
40i i i i i i i i 60i i i i i i i i 80i i s i i i s i 100i i s i i i s i 120i i s i i i s i 140i i–i i i–i
s Solubilization,i insolubilization;n(a-pinene):n(ILs):n(water)1:0.05:5
L.Li et al.
Table3Effect of the type of catalysts on the results of reaction
No.Catalysts Components of product(%)X(%)S(%)
a-Pinene Camphene Limonene Terpinolene a-Terpinol
1H2SO4(33%)0.34 5.4415.637.7911.2699.613.1 2H3PO40.57 4.5422.0232.5615.1499.317.7 3[Ac1820]?[H2PO4]-9.88 1.1912.411.2129.8765.237.3 4[Ac1820]?[HSO4]- 1.82 5.3613.3014.6245.9197.957.4
Reaction conditions:n(a-pinene):n(catalyst)n(water)1:0.05:5,a-pinene0.06mol,reaction temperature80°C, reaction time8h
X conversion of a-pinene,S selectivity of a-terpinol
Modi?ed ammonium salt ionic liquid
there is a absorption peak at1,460cm-1that means that the catalysts is of Lewis acid,and there is a absorption peak in1,537cm-1that means that the catalyst is of Bronsted acid[18].From Fig.2,it can be seen that the two catalysts are all of Lewis acid,and at the same time[Ac1820]?[HSO4]-is of Bronsted acid.It is suggested that the synergistic effect of Lewis and Bronsted acid for[Ac1820]?[HSO4]-can improve its catalytic activity.
L.Li et al.
The reusability of the[Ac1820]?[HSO4]-catalyst was tested at80°C.As shown in Fig.3,the conversion of a-pinene and selectivity to a-terpineol remained virtually identical during the recycle runs,the results indicated that[Ac1820]?[HSO4]-had good stability under reaction conditions.
Conclusion
The concept of CST for the Ac18n cationic ILs has been primarily applied to the hydration of a-pinene.It has been found that hydration of a-pinene catalyzed by [Ac1820]?[HSO4]-proceeds ef?ciently,and high conversion of a-pinene and yield of a-terpineol are obtained under the optimum reaction conditions.The [Ac1820]?[HSO4]-catalyst could be recycled up to four times and almost no loss in activity was observed.The new type ILs we developed based on the concept of CST provide not only a novel approach for the hydration of a-pinene but also a method for the water–organic matter reactions.
Acknowledgments The?nancial support provided for this research by the National Natural Science Foundation of China(31000275)and Postdoctoral Science Foundation of China(201104582)is gratefully acknowledged.
References
1.C.M.A′vila,https://www.360docs.net/doc/022309171.html,ellia,E.Rodriguez-Castellonc,J.Mol.Catal.A322,106(2010)
2.R.M.Traynor,R.M.Albert,R.L.Webb,in Naval Stores,ed.by D.F.Zinkel,J.Russels(Pulp
Chemical Association,New York,1989)
3.W.E.Erman,in Chemistry of the Monoterpenes,an Encyclopedic Handbook,ed.by D.Marcel
(Marcel Dekker,New York,1985)
4.M.C.Avila,https://www.360docs.net/doc/022309171.html,elli,E.N.Ponzi,M.I.Ponzi,XXI Simposio Ibero-Americano de Cata′lisis
(Ma′laga,2008)
5.J.E.Castanheiro,I.M.Fonseca,A.M.Ramos,R.Oliveira,Catal.Today104,296(2005)
6.J.E.Castanheiro,A.M.Ramos,I.Fonseca,J.Vital,Catal.Today82,187(2003)
7.D.P.A.Robles,K.A.D.Silva,M.R.H.Siddiqui,J.Mol.Catal.A175,33(2001)
8.A.M.Roma′n,S.Torre,F.W.Antu′nez,S.A.Robau,A.A.Elguezabal,Catal.Today107–108,310
(2005)
9.T.Mochida,R.Ohnishi,N.Horita,Y.Kamiya,T.Okuhara,Microporous Mesoporous Mater.101,
176(2007)
10.M.K.Yadav,M.V.Patil,R.V.Jasra,J.Mol.Catal.A297,101(2009)
11.R.L.Li,L.Zhao,J.Zheng,P.Li,Chin.Surf.Teter&Cosme34,293(2004)
12.Y.Zeng,Y.H.Wang,J.Y.Jiang,Z.L.Jin,https://www.360docs.net/doc/022309171.html,mun.19,70(2012)
13.N.Liu,C.Liu,Z.L.Jin,https://www.360docs.net/doc/022309171.html,an.Chem.13,2641(2011)
14.A.Behr,G.Henze,R.Schoma¨cker,Adv.Synth.Catal.348,1485(2006)
15.Z.L.Jin,N.Liu,C.Liu,Prog.Chem.22,1295(2010)
16.Y.H.Wang,J.Y.Jiang,X.W.Wu,F.Cheng,Z.L.Jin,Catal.Lett.79,55(2002)
17.S.Liu,L.Li,S.Yu,C.Xie,F.Liu,Z.Song,Chin.J.Catal.32,468(2011)
18.G.Yang,Y.Liu,Z.Zhou,Z.Zhang,Chem.Eng.J.168,351(2011)
19.J.Baret,F.Kleinschmidt,A.Harrak,Langmuir25,6088(2009)
20.Y.W.Fang,Y.J.Lu,J.F.Lv,X.M.Bie,Enzy.Microbial.Tech.44,84(2009)
常用法兰规格尺寸表
常用法兰规格尺寸表(国标) 发布时间:2010.07.13 新闻来源:法兰-法兰盘-法兰毛坯-弯头-三通-无缝钢管-聊城荣丰法兰制造厂浏览次数: 593 常用法兰规格尺寸表(国标) GB9119,2—88GB9119,2—88 in 法兰公称 通径 10kg=1.0MPa 公称通径 16kg=1.6MPa 法兰外 径 螺栓孔 距 螺栓直 径 螺栓孔数法兰厚度法兰外径螺栓孔距螺栓直径螺栓孔数法兰厚度 3/8 DN10 50 60 14 4 14 DN10 90 60 14 4 14 1/2 DN15 59 65 14 4 14 DN15 95 65 14 4 14 3/4 DN20 105 75 14 4 16 DN20 105 75 14 4 16 1 DN25 115 85 14 4 16 DN25 115 85 14 4 16 11/4DN32 140 100 18 4 18 DN32 140 100 18 4 18 11/2DN40 150 110 18 4 18 DN40 150 110 18 4 18 2 DN50 165 125 18 4 20 DN50 165 125 18 4 20 21/2DN65 185 145 18 4 20 DN65 185 145 18 4 20 3 DN80 200 160 18 8 20 DN80 200 160 18 8 20 31/2DN100 220 180 18 8 22 DN100 220 180 18 8 22 4 DN12 5 250 210 18 8 22 DN125 250 210 18 8 22 5 DN150 285 240 22 8 24 DN150 285 240 22 8 24 6 DN200 340 295 22 8 24 DN200 340 295 22 8 26 8 DN250 395 350 22 12 26 DN250 405 355 26 12 29 10 DN300 445 400 22 12 28 DN300 460 410 26 12 32 12 DN350 505 460 22 16 30 DN350 520 470 26 16 35 14 DN400 565 515 26 16 32 DN400 580 525 30 16 38 16 DN450 615 565 26 20 35 DN450 640 585 30 20 42 18 DN500 670 620 26 20 38 DN500 715 650 33 20 46 20 DN600 780 725 26 20 42 DN600 840 770 36 20 52
法兰盘规格尺寸
HG/T 20592 40-16 RF A=M20*1.5: 40-16是公称直径DN40的法兰,公称压力16公斤也就是1.6MPa,RF指密封面为突面,A=M20X1.5指该法兰上所配的螺栓规格。 GB9119,2—88GB9119,2—88 in 公称 通径 10kg=1.0MPa 公称 通径 16kg=1.6MPa 法 兰 外 径 螺栓 孔距 螺 栓 直 径 螺栓 孔数 法兰 厚度 法兰 外径 螺栓 孔距 螺栓 直径 螺栓 孔数 法兰厚度 3/8 DN10 506014414DN10 906014414 1/2 DN15 596514414DN15 956514414 3/4 DN20 1057514416DN20 1057514416 1 DN25 1158514416DN25 1158514416 11/4DN32 14010018418DN32 14010018418 11/2DN40 150********DN40 150******** 2 DN50 16512518420DN50 16512518420 21/2DN65 185********DN65 185******** 3 DN80 20016018820DN80 20016018820 31/2DN10 22018018822DN100 22018018822 4 DN12 5 25021018822DN125 25021018822 5 DN15 28524022824DN150 28524022824 6 DN20 34029522824DN200 34029522826 8 DN25 395350221226DN250 405355261229 10 DN30 445400221228DN300 460410261232 12 DN35 505460221630DN350 520470261635
法兰盘规格尺寸
GB9119,2—88GB9119,2—88 in 公称 通径 10kg=1.0MPa 公称 通径 16kg=1.6MPa 法 兰 外 径 螺栓 孔距 螺 栓 直 径 螺栓 孔数 法兰 厚度 法兰 外径 螺栓 孔距 螺栓 直径 螺栓 孔数 法兰厚度 3/8 DN10 506014414DN10 906014414 1/2 DN15 596514414DN15 956514414 3/4 DN20 1057514416DN20 1057514416 1 DN25 1158514416DN25 1158514416 11/4DN32 14010018418DN32 14010018418 11/2DN40 150********DN40 150******** 2 DN50 16512518420DN50 16512518420 21/2DN65 185********DN65 185******** 3 DN80 20016018820DN80 20016018820 31/2DN10 22018018822DN100 22018018822 4 DN12 5 25021018822DN125 25021018822 5 DN15 28524022824DN150 28524022824 6 DN20 34029522824DN200 34029522826 8 DN25 395350221226DN250 405355261229 10 DN30 445400221228DN300 460410261232 12 DN35 505460221630DN350 520470261635 14 DN40 565515261632DN400 580525301638 16 DN45615565262035DN450 640585302042
常用法兰规格尺寸表
常用法兰规格尺寸表(国标、美标、日标) GB9119,2—88 GB9119,2—88 in 公称通径 10kg=1.0MPa 公称通径 16kg=1.6MPa 法兰外径 螺栓孔距 螺栓直径 螺栓孔数 法兰厚度 法兰外径 螺栓孔距 螺栓直径 螺栓 孔数 法兰厚度 3/8 DN10 50 60 14 4 14 DN10 90 60 14 4 14 ㎡㎏ 1/2 DN15 59 65 14 4 14 DN15 95 65 14 4 14 3/4 DN20 105 75 14 4 16 DN20 105 75 14 4 16 1 DN25 115 85 14 4 16 DN2 5 115 85 14 4 1 6 11 /4 DN32 140 100 18 4 18 DN32 140 100 18 4 18 11 /2 DN40 150 110 18 4 18 DN40 150 110 18 4 18 2 DN50 165 125 18 4 20 DN50 165 125 18 4 20 21/2 DN65 185 145 18 4 20 DN65 185 145 18 4 20 3 DN80 200 160 18 8 20 DN80 200 160 18 8 20 31/2 DN100 220 180 18 8 22 DN100 220 180 18 8 22 4 DN125 250 210 18 8 22 DN125 250 210 18 8 22 5 DN150 285 240 22 8 24 DN150 285 240 22 8 24 6 DN200 340 295 22 8 24 DN200 340 295 22 8 26 8 DN250 395 350 22 12 26 DN250 405 355 26 12 29 10 DN300 445 400 22 12 28 DN300 460 410 26 12 32 12 DN350 505 460 22 16 30 DN350 520 470 26 16 35 14 DN400 565 515 26 16 32 DN400 580 525 30 16 38 16 DN450 615 565 26 20 35 DN450 640 585 30 20 42 18 DN500 670 620 26 20 38 DN500 715 650 33 20 46 20 DN600 780 725 26 20 42 DN600 840 770 36 20 52 24 JIS 标准 JIS 标准 JIS 标准 in 公称通径 10kg=1.0MPa 公称通 径 16kg=1.6MPa 公称通径 20kg=2.0MPa 法兰外径 螺栓孔距 螺栓直径 螺栓孔数 法兰厚度 法兰外径 螺栓孔距 螺栓直径 螺栓 孔数 法兰厚度 法兰外径 螺栓孔距 螺栓直径 螺 栓孔 数 法兰厚度 3/8 DN10 DN10 DN10 1/2 DN15 DN15 DN15 3/4 DN20 DN20 DN20 1 DN25 DN25 DN25 11 /4 DN32 DN32 DN32 11/2 DN40 140 105 19 4 16 DN40 140 105 19 4 16 DN40 140 105 19 4 18 2 DN50 155 120 19 4 16 DN50 155 120 19 8 16 DN50 155 120 19 8 18 21 /2 DN65 175 140 19 4 18 DN65 175 140 19 8 18 DN65 175 140 19 8 20 3 DN80 185 150 19 8 18 DN80 200 , 160 22 8 20 DN80 200 160 23 8 22 31/2 DN90 195 160 19 8 18 DN90 210 170 22 8 20 DN90 210 170 23 8 24
