Chronic high glucose

Chronic high glucose downregulates mitochondrial calpain 10and contributes to renal cell death and diabetes-induced renal injury
Marisa D.Covington 1and Rick G.Schnellmann 1,2
1
Center for Cell Death,Injury and Regeneration,Department of Pharmaceutical and Biomedical Sciences,Medical University of South Carolina,Charleston,South Carolina,USA and 2Ralph H.Johnson Veterans Administration Medical Center,Charleston,South Carolina,USA
Whereas most calpains are cytosolic proteases,calpain 10is resident in mitochondria and is important in mitochondrial homeostasis.Because calpain 10has been implicated in type 2diabetes,we studied its possible role in diabetes-induced renal dysfunction.We treated renal proximal tubular cells with high glucose (17mmol/l)and found decreased mitochondrial calpain 10mRNA and protein at 96h
compared with cells incubated with 0or 5mmol/l glucose or 17mmol/l D -mannitol.High glucose increased mitochondrial calpain 10substrates (NDUFB8and ATP synthase b ),
decreased basal and uncoupled respiration,and initiated cell apoptosis as indicated by cleaved caspase 3and nuclear condensation.Renal calpain 10protein and mRNA were specifically decreased in streptozotocin-induced diabetic rats with kidney dysfunction,and in diabetic ob/ob mice.In agreement with our in vitro data,the kidneys of
streptozotocin-induced diabetic rats had elevated calpain 10substrates and cleaved caspase 3.Finally,specific siRNA-induced knockdown of calpain 10in the proximal tubules of control rats resulted in decreased renal function as evidenced by increased serum creatinine,and increased caspase 3cleavage compared with rats receiving scrambled siRNA.Thus,the glucose-induced loss of calpain 10in vivo results in renal cell apoptosis and organ failure through accumulation of mitochondrial calpain 10substrates and mitochondrial dysfunction.Whether this is a major cause of the decreased renal function in diabetic nephropathy will require further studies.
Kidney International (2012)81,391–400;doi:10.1038/ki.2011.356;published online 19October 2011
KEYWORDS:acute kidney injury;apoptosis;diabetic nephropathy;hyperglycemia;mitochondria
The most prevalent cause of chronic renal failure and end-stage renal disease is diabetic nephropathy—previously described as a glomerulopathy associated with diffuse or nodular glomerulosclerosis—although fewer than one-third of diabetic patients have this type of glomerulopathy.1,2Proximal tubular functional and structural changes correlate better with diabetic nephropathy progression,and may be key to kidney dysfunction development in diabetes.3,4
Disruption of mitochondrial function is important in various diseases including diabetes,and diabetes induced in rats by streptozotocin (STZ)or alloxan treatment results in impaired mitochondrial respiration and disruption of energy production and mitochondrial protein synthesis in the kidney.5–8Ultimately,changes in mitochondrial viability lead to cell death via apoptosis or necrosis,but the exact mechanism of mitochondrial dysfunction in diabetic nephropathy is unclear.
Calpain 10is a ubiquitously expressed ‘atypical’calpain.9,10Calpain 10gained much attention following a genome-wide linkage scan for susceptibility genes associated with dia-betes.11,12Since calpain 10was identi?ed as a type 2diabetes susceptibility gene in 2000,there have been numerous reports supporting and refuting this proposed association.11,13Never-theless,calpain 10has been implicated in both insulin-stimulated glucose uptake 14,15and insulin secretion 16,17in islet cells.
Whereas calpains were traditionally thought to be cytosolic proteases,our laboratory identi?ed calpain 10as the resident mitochondrial calpain in isolated rabbit,mouse,and rat renal mitochondria with primary activity in the matrix.18,19In addition,calpain 10was shown to be a mediator of Ca 2t-induced mitochondrial dysfunction through cleavage of complex I subunits,NDUFV2and NDUFB8,of the electron transport chain.18Interestingly,overexpression of calpain 10in NIH-3T3cells resulted in mitochondrial swelling,autophagy,and cell death,18suggest-ing that calpain 10is important in mitochondrial homeo-stasis.In contrast,knockdown of calpain 10protein expression resulted in renal proximal tubular cell (RPTC)apoptosis,20illustrating the double-edged sword of calpain 10.
https://www.360docs.net/doc/6512369707.html, o r i g i n a l a r t i c l e
&2012International Society of Nephrology
Received 1June 2010;revised 1September 2011;accepted 7September 2011;published online 19October 2011
Correspondence:Rick G.Schnellmann,Center for Cell Death,Injury and Regeneration,Department of Pharmaceutical and Biomedical Sciences,Medical University of South Carolina,280Calhoun Street POB 250140,Charleston,South Carolina 29425,USA.E-mail:schnell@https://www.360docs.net/doc/6512369707.html,
We also observed a decrease in renal calpain10protein and
mRNA expression in aged humans,mice,and rats that was
attenuated with caloric restriction and correlated with
decreased kidney function in rats.20These studies provide
evidence that renal calpain10is important in age-related loss
of renal function.The goal of this study was to determine the
role of calpain10in renal dysfunction observed in diabetes
using two different animal models of diabetes:an RPTC
in vitro model and an in vivo rat kidney model after calpain10
knockdown.
RESULTS
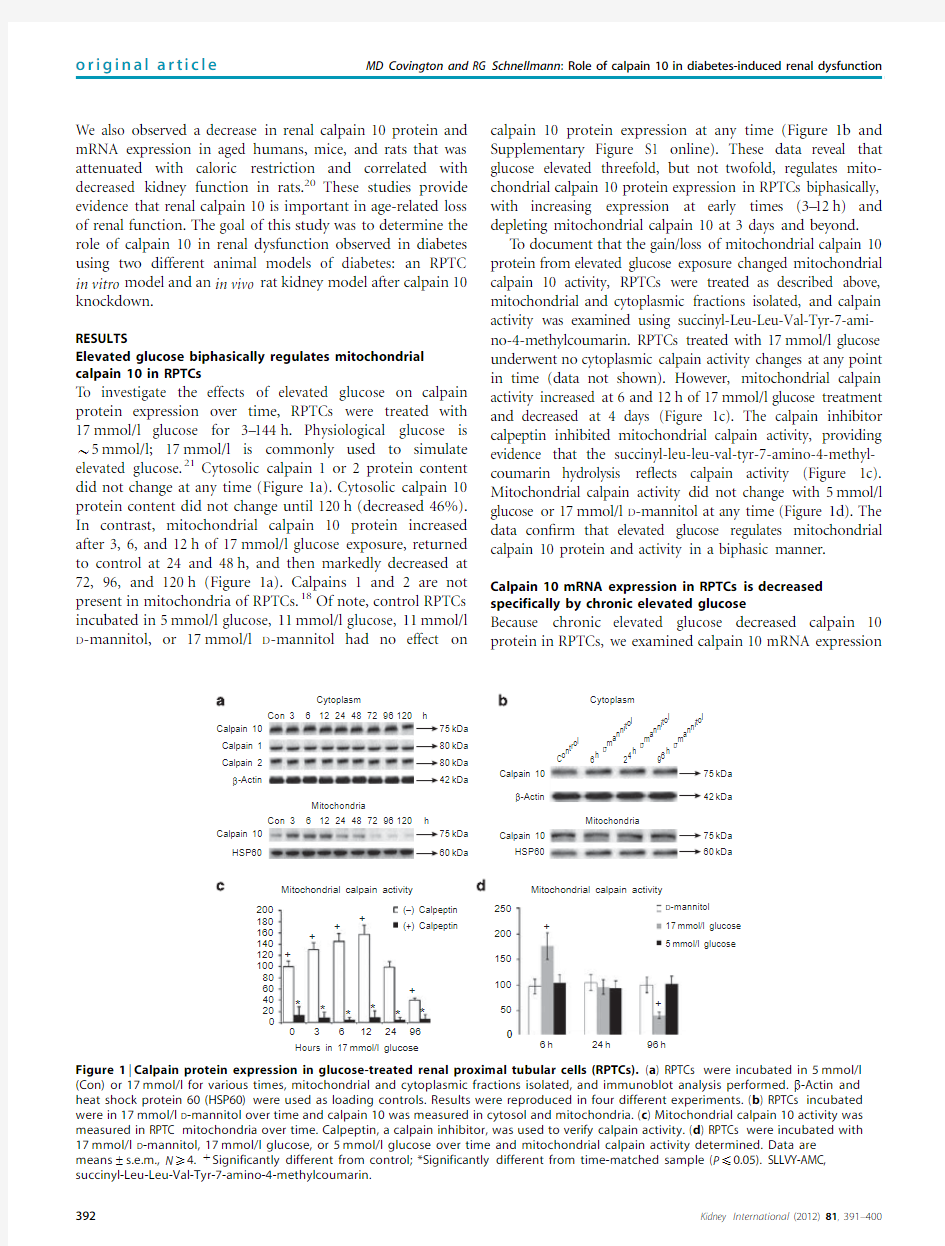
Elevated glucose biphasically regulates mitochondrial calpain10in RPTCs
To investigate the effects of elevated glucose on calpain
protein expression over time,RPTCs were treated with
17mmol/l glucose for3–144h.Physiological glucose is B5mmol/l;17mmol/l is commonly used to simulate elevated glucose.21Cytosolic calpain1or2protein content
did not change at any time(Figure1a).Cytosolic calpain10
protein content did not change until120h(decreased46%).
In contrast,mitochondrial calpain10protein increased
after3,6,and12h of17mmol/l glucose exposure,returned
to control at24and48h,and then markedly decreased at
72,96,and120h(Figure1a).Calpains1and2are not
present in mitochondria of RPTCs.18Of note,control RPTCs
incubated in5mmol/l glucose,11mmol/l glucose,11mmol/l D-mannitol,or17mmol/l D-mannitol had no effect on calpain10protein expression at any time(Figure1b and Supplementary Figure S1online).These data reveal that glucose elevated threefold,but not twofold,regulates mito-chondrial calpain10protein expression in RPTCs biphasically, with increasing expression at early times(3–12h)and depleting mitochondrial calpain10at3days and beyond.
T o document that the gain/loss of mitochondrial calpain10 protein from elevated glucose exposure changed mitochondrial calpain10activity,RPTCs were treated as described above, mitochondrial and cytoplasmic fractions isolated,and calpain activity was examined using succinyl-Leu-Leu-Val-Tyr-7-ami-no-4-methylcoumarin.RPTCs treated with17mmol/l glucose underwent no cytoplasmic calpain activity changes at any point in time(data not shown).However,mitochondrial calpain activity increased at6and12h of17mmol/l glucose treatment and decreased at4days(Figure1c).The calpain inhibitor calpeptin inhibited mitochondrial calpain activity,providing evidence that the succinyl-leu-leu-val-tyr-7-amino-4-methyl-coumarin hydrolysis re?ects calpain activity(Figure1c). Mitochondrial calpain activity did not change with5mmol/l glucose or17mmol/l D-mannitol at any time(Figure1d).The data con?rm that elevated glucose regulates mitochondrial calpain10protein and activity in a biphasic manner. Calpain10mRNA expression in RPTCs is decreased specifically by chronic elevated glucose
Because chronic elevated glucose decreased calpain10 protein in RPTCs,we examined calpain10mRNA
expression
Con
Calpain 1
Calpain 10
Calpain 10
Calpain 10
kDa
kDa
kDa
Calpain 10
(+) Calpeptin
+
+
++
+
+
+
*
*
*
*
*
*
20
40
60
80
100
120
140
S
L
L
V
Y
-
A
M
C
c
l
e
a
v
a
g
e
%
o
f
c
o
n
t
r
o
l
160
180
200
HSP60
HSP60
Calpain 2
β-Actin
β-Actin
612244872
Cytoplasm Cytoplasm
h
h
kDa
kDa
kDa
kDa
kDa
kDa C o
n
t
r
o
l
6
h
D
-
m
a
n
n
i
t
o
l
2
4
h
D
-
m
a
n
n
i
t
o
l
9
6
h
D
-
m
a
n
n
i
t
o
l
kDa
Mitochondria
Mitochondria
96120
3
Con612244872
Mitochondrial calpain activity
0361296
24
Hours in 17mmol/l glucose
(–) Calpeptin250
Mitochondrial calpain activity
D-mannitol
17mmol/l glucose
5mmol/l glucose
200
%
C
o
n
t
r
o
l
o
f
c
a
l
p
a
i
n
a
c
t
i
v
i
t
y
150
100
50
6h24h96h
96120
3
Figure1|Calpain protein expression in glucose-treated renal proximal tubular cells(RPTCs).(a)RPTCs were incubated in5mmol/l (Con)or17mmol/l for various times,mitochondrial and cytoplasmic fractions isolated,and immunoblot analysis performed.b-Actin and heat shock protein60(HSP60)were used as loading controls.Results were reproduced in four different experiments.(b)RPTCs incubated were in17mmol/l D-mannitol over time and calpain10was measured in cytosol and mitochondria.(c)Mitochondrial calpain10activity was measured in RPTC mitochondria over time.Calpeptin,a calpain inhibitor,was used to verify calpain activity.(d)RPTCs were incubated with 17mmol/l D-mannitol,17mmol/l glucose,or5mmol/l glucose over time and mitochondrial calpain activity determined.Data are
means±s.e.m.,N X4.tSignificantly different from control;*Significantly different from time-matched sample(P p0.05).SLLVY-AMC, succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin.
to determine if this decrease was transcriptionally regulated.Calpain 1mRNA expression did not change at any time in 17mmol/l glucose-treated RPTCs (Figure 2).However,cal-pain 10mRNA expression decreased,but only after 4days of elevated glucose exposure (Figure 2,data not shown),correlating with the decrease in calpain 10protein and activity.No change in calpain 10mRNA occurred in cells incubated with 17mmol/l D -mannitol.Thus,prolonged incubation with elevated glucose transcriptionally down-regulates calpain 10in RPTCs.
Mitochondrial function is decreased in RPTCs exposed to chronic elevated glucose
Because chronic elevated glucose caused loss of mito-chondrial calpain 10expression,RPTCs were treated with 17mmol/l glucose and RPTC respiration was measured at different times.There was no change in basal respiration until 4days of glucose treatment at which time it decreased 50%(Figure 3a).After basal respiration was determined,FCCP (carbonyl cyanide-p -tri?uoromethoxyphenylhydrazone)was added to obtain uncoupled QO 2,which is used here as a stress test to ascertain underlying defects in the electron transport chain when maximally stimulated.Uncoupled respiration remained constant until 4days of glucose treatment at which time it decreased 50%(Figure 3b).Basal
or uncoupled respiration did not change at any time in RPTCs treated with 17mmol/l D -mannitol (Figure 3a and b).Therefore,prolonged exposure to elevated glucose causes mitochondrial dysfunction in RPTCs.
Prolonged exposure to glucose elevates protein expression of NDUFB8and ATP synthase b
We previously reported that electron transport chain protein NDUFB8and adenosine triphosphate (ATP)synthase b are substrates for mitochondrial calpain 10.18Because 17mmol/l glucose decreased mitochondrial calpain 10expression,we hypothesized that mitochondrial calpain 10substrates may increase.With immunoblot,we noted no change in NDUFB8or ATP synthase b protein expression in 17mmol/l glucose-treated RPTCs at 6or 24h (Figure 4a).However,both proteins increased after 96h of glucose treatment.Accumula-tion of NDUFB8and ATP synthase b correlates with the loss of mitochondrial calpain 10at 96h.There was no change in NDUFB8or ATP synthase b in RPTCs treated with 17mmol/l D -mannitol (Figure 4b).In addition,mRNA of NDUFB8or ATP synthase b did not change,suggesting that increases in these proteins are not because of increased transcription under these conditions (Figure 4c and
d).
Glucose treatment
6
24
96
h
Calpain 10
Calpain 1
β-Actin
Calpain 10 mRNA
C a l p a i n 10 m R N A β-A c t i n (f o l d o f c o n t r o l )
1.4
17mmol/l glucose 17mmol/l mannitol
1.2
10.80.60.40.20
6
Hours
24
96
*
Figure 2|Calpain mRNA expression in glucose-treated renal proximal tubular cells (RPTCs).RPTCs were incubated in
17mmol/l glucose or 17mmol/l D -mannitol over time.Cells were collected,mRNA was isolated,and reverse transcriptase-PCR (RT-PCR)was performed using calpain 10,calpain 1,and b -actin primers.Data are means ±s.e.m.,N X 4.*Significantly different from D -mannitol (P p 0.05).
40506040708017mmol/l glucose
17mmol/l D -mannitol
17mmol/l glucose 17mmol/l D -mannitol
B a s a l r e s p i r a t i o n (n m o l O 2/m i n /m g )
F C C P -u n c o u p l e d r e s p i r a t i o n (n m o l O 2/m i n /m g )
Basal respiration
4896
Hours
Hours
*
*
Uncoupled respiration
243530302020250
12
4896
240
12
15101050
Figure 3|Mitochondrial respiration in glucose-treated renal proximal tubular cells (RPTCs).RPTCs were incubated in
17mmol/l glucose or 17mmol/l D -mannitol over time.Cells were collected and (a )basal and (b )uncoupled respiration was
measured.Uncoupled respiration was measured in the presence of 10m mol/l carbonyl cyanide-p -trifluoromethoxyphenylhydra-zone (FCCP).Data are means ±s.e.m.(N ?4).*Significantly different from D -mannitol (P p 0.05).
Chronic elevated glucose causes RPTC apoptosis
We previously showed that ‘knockdown’of mitochondrial calpain 10caused RPTC apoptosis.20To investigate the effects of chronic elevated glucose on RPTC apoptosis,we examined nuclear condensation and caspase 3activation.Examination of nuclear morphology using 4,6-diamidino-2-phenylindole staining revealed normal nuclei (495%)in controls and at 6h of 17mmol/l glucose treatment (Figure 5a and b).However,at 96h of glucose treatment,RPTCs contained B 30%condensed https://www.360docs.net/doc/6512369707.html,ing immunoblot analysis,we observed cleaved procaspase 3in RPTCs treated with 17mmol/l glucose for 96h (Figure 5c).No cleaved procas-pase 3was observed in control and RPTCs treated with glucose for 6h (Figure 5c).Thus,loss of calpain 10by chronic elevated glucose causes RPTC apoptosis.
Renal calpain 10protein expression is depleted in STZ-induced diabetic rats and diabetic ob/ob mice
Our RPTC data revealed that chronic elevated glucose decreased calpain 10expression,and hence we next measured calpain expression in two in vivo rodent diabetes models.At 10weeks after induction of STZ-induced diabetes in the rat,serum glucose was 408±30mg/dl and serum creatinine was 2.8±0.5mg/dl.In contrast,control,vehicle-treated animals had serum glucose of 128±17mg/dl and serum creatinine of 0.5±0.1mg/dl.Thus,this diabetic rat model has increased serum glucose and decreased kidney function at https://www.360docs.net/doc/6512369707.html,pared with controls,renal calpain 1or 2protein expression did not change in STZ-treated rats (Figure 6a).In contrast,calpain 10protein expression decreased in STZ-induced rats,suggesting that the effect of diabetes was unique
to calpain https://www.360docs.net/doc/6512369707.html,pared with controls,calpain 10mRNA was also decreased,suggesting that calpain 10is transcriptionally downregulated in STZ-induced diabetic kidneys (Figure 6b).Calpain 1mRNA did not change,correlating with the lack of change in calpain 1protein expression (Figure 6b).
We also measured renal calpain 10in another in vivo model of diabetes,the ob/ob mouse.We obtained blood and kidney samples from both control and ob/ob 8-week-old male mice.The average blood glucose in the ob/ob mice was 400±31mg/dl and 204±29mg/dl in lean mice.There was a decrease in renal calpain 10protein expression in the diabetic ob/ob mice compared with the control but no change in calpain 1protein expression (Figure 6c).These results reveal that calpain 10is speci?cally decreased in two in vivo models of diabetes.Importantly,in the STZ model,we observed a decrease in renal calpain 10protein and mRNA expression that correlates with decreased renal function.In addition,renal calpain 10is transcriptionally downregulated in STZ-induced diabetic rats.
NDUFB8and ATP synthase b protein expression and apoptosis increase in STZ-induced diabetic kidneys
Because we observed increases in the calpain 10substrates NDUFB8and ATP synthase b in RPTCs treated chronically with elevated glucose,we examined the expression of these proteins in STZ-induced diabetic https://www.360docs.net/doc/6512369707.html,pared with controls,there was an increase in both NDUFB8and ATP synthase b protein expression in the STZ-induced diabetic kidney,correlating with our in vitro data (Figure 7a and b).In addition,cleaved procaspase 3and increased terminal deoxynucleotidyl transferase dUTP nick end
labeling
17mmol/l glucose-treated RPTCs Con a
c
d
b
NDUFB8NDUFB8NDUFB8 mRNA
ATP synthase β mRNA 1.6
1.41.20.60.40.20
624Hours
960
624Hours
96
17mmol/l glucose 17mmol/l mannitol
N D U F B 8 m R N A /β-A c t i n
(f o l d o f c o n t r o l )
A T P s y n t h a s e β m R N A /β-A c t i n (f o l d o f c o n t r o l )
0.81 1.61.41.20.60.40.20
0.81β-Actin
β-Actin
ATP synthase β
C
o n
t r o
l 6
h D -m a n n i t o l 24h D -m a n n i t o l 96h D -m a n n i t o l ATP
synthase β
62496
55kDa 19kDa 42kDa
55kDa 19kDa 42kDa
17mmol/l glucose 17mmol/l mannitol
Figure 4|NDUFB8and adenosine triphosphate (ATP)synthase b protein expression in glucose-treated renal proximal tubular cells (RPTCs).RPTCs were incubated in (a )17mmol/l glucose or (b )17mmol/l D -mannitol over time.Cell lysates were subjected to immunoblot analysis for NDUFB8and ATP synthase b .b -Actin was used as a loading control.mRNA was isolated and reverse transcriptase-PCR (RT-PCR)was performed using (c )NDUFB8and (d )ATP synthase b primers.Data are means ±s.e.m.(N ?4).
Calpain 1Calpain 1
Calpain 10
Calpain 10a
b
kDa
kDa
kDa
kDa
Calpain 2β-Actin
β-Actin
S T Z
C
o n
t r o
l
S T Z
C
o n
t r o
l
S T Z
C
o n
t r o
l S T Z
C o n
t r o
l
c
Calpain 1Calpain 10kDa kDa kDa
β-Actin
O
b /
o b
C
o n
t r o l Ob/ob mice Figure 6|Calpain expression in kidneys of diabetic models.Sprague–Dawley rats,8weeks of age (200–250g),were starved for 16h and injected once into the tail vein with streptozotocin (STZ;55mg/kg)in sodium citrate buffer.At 10weeks after induction of diabetes,rats were killed and
blood and kidneys were harvested.Renal cell lysates and mRNA were isolated.(a )Immunoblot analysis for calpains 10,1,and 2was performed.(b )Reverse transcriptase-PCR (RT-PCR)analysis for calpains 10and 1was performed.(c )Immunoblot analysis was performed on ob/ob mice kidney samples.Results were reproduced in at least four different animals.
Con 4030352520151050
Con
Cleaved caspase 3
Caspase 3
β-Actin
36kDa 17kDa 42kDa Con
6 h 6 h
6 h
Glucose (17mmol/l)*
96 h
96 h
96 h
% C o n d e n s e d n u c l e i
Figure 5|Apoptotic cell death in glucose-treated renal proximal tubular cells (RPTCs).RPTCs were incubated in 17mmol/l over time.Apoptosis was measured by (a,b )nuclear condensation and (c )procaspase 3cleavage.(a )Nuclei were identified by 4,6-diamidino-2-phenylindole (DAPI)staining and visualized using a Nikon TE300Eclipse Fluorescence microscope (Nikon,Melville,NY)with excitation and emission filters of 350and 486nm,respectively.(b )Condensed nuclei from 10non-overlapping fields were counted for each treatment group.Data are means ±s.e.m.,N ?4.*Significantly different from control and 6h (P o 0.05).(c )Cell lysates were subjected to immunoblot analysis for procaspase 3and cleaved caspase 3(17kDa).b -Actin was used as a loading control.
(TUNEL)staining were observed in the STZ-induced diabetic kidney,providing evidence that STZ-induced diabetes is causing renal apoptosis (Figure 7a–c).There was no change in mRNA expression of NDUFB8and ATP synthase b (Figure 7d),correlating with our in vitro data and providing evidence that the increase in NDUFB8and ATP synthase b is not due to increased synthesis.
In vivo siRNA knockdown of renal calpain 10
To determine whether decreased calpain 10is suf?cient to induce renal dysfunction,rats were treated with calpain 10small interfering RNA (siRNA)to decrease renal calpain 10.This effective approach has been used by many investigators because siRNA accumulates in the renal proximal tubule and decreases its target protein.22–26Rats were treated with siRNA (20nmol)directed against calpain 10or scrambled siRNA by tail vein injection.Tissues were isolated,homogenized,and immunoblot analysis performed.A single intravenous dose of a siRNA directed against calpain 10,but not that of a negative siRNA (scrambled),decreased calpain 10protein and mRNA expression in rat kidney cortex (Figure 8a).The siRNA directed against calpain 10had no effect on calpain 1in kidney cortex or calpain 10in the liver (Supplementary Figure S2online).Therefore,we have an in vivo model of renal-speci?c calpain 10knockdown to examine the functional consequences thereof.
To examine the effects of calpain 10loss in rat kidneys,we measured serum creatinine,cleavage of caspase 3,and TUNEL staining.In rats treated with the calpain 10siRNA,there was an increase in serum creatinine to 1.7±0.2mg/dl
at 7days (Figure 8b).The serum creatinine for negative siRNA was 0.7±0.1mg/dl and did not change over time.These data suggest that the loss of renal calpain 10causes renal dysfunction.In addition,immunoblots of caspase 3showed activation of caspase 3after 5and 7days of calpain 10siRNA but not in negative siRNA-treated rat kidneys (Figure 8c).There was also an increase in TUNEL-positive cells in the calpain 10siRNA-treated kidney (Figure 8d).We suggest that the loss of calpain 10in vivo results in renal apoptosis and renal dysfunction.
DISCUSSION
Our data reveal that diabetic glucose speci?cally decreases mitochondrial calpain 10expression in RPTCs and that renal calpain 10is decreased in diabetic rats and mice.Further-more,the loss of renal calpain 10resulted in apoptosis in vitro and in vivo ,and decreased renal function in the rat.We suggest that the loss of calpain 10is key in diabetes-induced renal dysfunction and,ultimately,diabetic nephrop-athy.These data complement our previous work in which we reported that mitochondrial calpain 10is required for cell viability,and calpain 10speci?cally decreases in aging rat,mouse,and human kidney tissues when renal function decreases.20We provide evidence that calpain 10is required for renal function and contributes to two common renal diseases.Finally,calpain 10is ubiquitously expressed and our ?ndings have broader implications in other tissues and diseases.
Examining the effect of elevated glucose on calpain expression over time,we observed an acute increase
in
S T Z C
o n t r o l S T Z
C o n t r o l STZ
Control Caspase 3
β-Actin
β-Actin
β-Actin NDUFB8kDa kDa kDa kDa
*
8070605040203010T U N E L -p o s i t i v e c e l l s
kDa kDa kDa 350STZ-induced diabetic rats
*
*
*
300200150100ATP synthase βNDUFB8
1.4
ATP synthase βNDUFB8
Con Control STZ
STZ 1.210.40.60.8m R N A /β-A c t i n
(f o l d o f c o n t r o l )
0.20
250500
P r o t e i n e x p r e s s i o n % c o n t r o l
ATP synthase β
Caspase 3Cleaved caspase 3
Figure 7|NDUFB8,adenosine triphosphate (ATP)synthase b ,and caspase 3protein expression in streptozotocin (STZ)-induced diabetic kidneys.STZ-induced diabetic kidneys were isolated and the tissue was homogenized.Immunoblot analysis for b -actin,NDUFB8,ATP synthase b ,procaspase 3,and cleaved caspase 3(17kDa)was (a )performed and (b )quantified (open bars are control animals and black bars are STZ-treated animals).(c )Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells were determined.(d )NDUFB8and ATP synthase b mRNA was examined using reverse transcriptase-PCR (RT-PCR).Results were reproduced in at least four different animals.Data are means ±s.e.m.,N X 4.*Significantly different from control (P o 0.05).
calpain 10protein and activity between 3and 12h after 17mmol/l glucose exposure that returned to control at 24h.Increased calpain 10was not due to increased transcription and we propose that increased calpain 10is due to decreased degradation.Finally,the rapid increase in calpain 10by glucose suggests that renal mitochondrial calpain 10is important in renal metabolism and is regulated by nutrients.
It is noteworthy that the acute and chronic effects of glucose act speci?cally on mitochondrial calpain 10;two calpain family members (calpains 1and 2)did not change over time.In contrast,the decrease in calpain 10protein and activity in RPTCs after 3–5days of 17mmol/l glucose treatment was associated with a decrease in calpain 10mRNA,suggesting glucose induced a decrease in calpain 10transcription.A mechanism for decreased calpain 10transcription by glucose has not been reported.
The decrease in mitochondrial calpain 10protein occurred before a decrease in cytoplasmic calpain 10.We reported similar results when calpain 10was ‘knocked down’with short hairpin RNA 20and suggest that mitochondrial calpain 10turnover is greater than cytosolic calpain 10.
The decrease in RPTC calpain activity observed during chronic glucose exposure also has been reported in other cellular models.Diaz-Villasenor et al .27reported a decrease in calpain activity in lymphocytes from diabetic patients.The increase in RPTC calpain activity by short-term glucose also has been reported to occur in cardiomyocytes,28but the calpain isoform that was being measured was not identi?ed.
Given that calpain 10is a protease,decreased calpain 10expression may result in the accumulation of its substrates.There was no change in NDUFB8and ATP synthase b after acute glucose treatment of RPTCs,but chronic glucose-induced RPTC mitochondrial calpain 10loss resulted in increased NDUFB8and ATP synthase b .We also explored this possibility in the rat STZ diabetic model and observed that renal NDUFB8and ATP synthase b increased in concert with the loss of renal calpain 10.Thus,we hypothesize that the initiating mitotoxic event induced by the loss of mitochondrial calpain 10is the accumulation of mitochondrial calpain 10substrates.Supporting our hypoth-esis,we observed that chronic glucose treatment decreased RPTC basal and uncoupled RPTC respiration,markers of mitochondrial dysfunction.We observed no changes in mitochondrial function at earlier times of glucose treatment (that is,12,24,and 48h).
We previously reported that knockdown of calpain 10with short hairpin RNA resulted in RPTC apoptosis.20We observed apoptosis at 96h after glucose treatment,when calpain 10was decreased,but not at 6h.We also explored this possibility in the rat STZ diabetic model and observed caspase 3cleavage in concert with the loss of renal calpain 10.Importantly,we show the loss of renal calpain 10by siRNA in vivo results in activation of caspase 3in the kidney cortex.These results combined with the results from our earlier study 20provide evidence that the accumulation of mitochondrial proteins and mitochondrial
dysfunction
2.5211.50.50
5 Days
7 Days
Calpain 10 siRNA *
*
Negative siRNA
S e r u m c r e a t i n i n e (m g /d l )
2 Days
Days of siRNA treatment
Kidney cortex Neg siRNA a
c
d
b
Neg siRNA Neg siRNA
Cal 10 siRNA
Cal 10 siRNA
Cal 10 siRNA
Calpain 10Calpain 1kDa 4540
3530252015100
5T U N E L -p o s i t i v e c e l l s
kDa kDa
Cleaved caspase 3Caspase 3Calpain 10Day
2
2
5
7
7
75kDa 42kDa
5
Day
2
2
5
7
7
5
GAPDH
GAPDH
GAPDH
Kidney cortex mRNA
++++++
––––––++++++––––––Neg siRNA Cal 10 siRNA Day
22577
5++++++––––––Figure 8|The effect of small interfering RNA (siRNA)on calpain protein in rat kidney.Eight-week-old rats were treated with 20nmol of siRNA directed against calpain 10(Cal 10)or a scrambled siRNA (Neg)by tail vein injection.(a )Kidney cortex was isolated and calpain 10protein was measured by immunoblot analysis.Calpain 10and 1mRNA was measured by reverse transcriptase-PCR (RT-PCR).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)served as a loading control.(b )Blood was collected from rats and serum creatinine was determined.(c )Kidney cortex was isolated and procaspase 3and cleaved caspase 3(17kDa)protein was measured by immunoblot analysis.GAPDH served as a loading control.(d )Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells were determined.Results were reproduced in at least four different animals.Data are means ±s.e.m.,N X 4.*Significantly different from control (P o 0.05).
associated with the loss of renal calpain10lead to apoptosis in vitro and in vivo.
In an earlier study,25mmol/l glucose for6and24h produced necrotic cell death in LLC-PK cells.29However,we observed no RPTC death at6or24h.These results could be due to different glucose concentrations used but,more likely,the difference arises from the use of an immortalized cell line and our use of primary cultures cultured under aerobic conditions.30,31
STZ-induced diabetic rats had elevated glucose and increased serum creatinine after10weeks.As we observed in RPTCs,renal calpain10mRNA and protein were decreased and calpains1and2were not,suggesting that decreases in renal calpain10observed in diabetes is because of decreased calpain10transcription.We conducted a similar experiment in the diabetic ob/ob mouse and observed that with elevated glucose,renal function decreased,suggesting that calpain10decreases with elevated glucose in multiple animal models.
We developed an in vivo model of renal calpain10 knockdown using siRNA.This effective approach has been used by a number of investigators:siRNA accumulates in the renal proximal tubule and decreases its target protein.22–26 The loss of both protein and mRNA expression of calpain 10in the kidney cortex results in kidney dysfunction.Caspase 3activation increased,suggesting that the loss of calpain 10in vivo results in apoptosis.These data provide strong evidence that the loss of renal calpain10is suf?cient to induce kidney injury.
In summary,renal calpain10protein and mRNA decrease with chronic elevated glucose,both in vitro and in vivo,a decrease that correlates with increased calpain10substrates resulting in mitochondrial dysfunction and apoptosis.Loss of renal calpain10by siRNA in vivo results in renal dysfunction and apoptosis,suggesting a direct relationship between loss of calpain10protein expression and renal dysfunction.These data collectively indicate that the loss of calpain10in vivo results in renal apoptosis and renal dysfunction,underscoring that the loss of calpain10causes mitochondrial dysfunction leading to cell death in diabetic nephropathy.
MATERIALS AND METHODS
Renal proximal tubules
Isolation and culture of renal proximal tubules were performed as described previously.30,31RPTCs were isolated from female New Zealand White rabbits(1.5–2.5kg)using the iron oxide perfusion method and grown under improved conditions.The advantage of this model is that RPTCs cultured under these conditions exhibit greater differentiated function,an in vivo–like rate of oxidative metabolism,and limited glycolysis and are gluconeogenic.30,31 Mitochondrial isolation and fractionation
RPTC mitochondria were isolated via differential centrifugation as previously described.18Mitochondrial purity was determined by measuring the levels of glyceraldehyde3-phosphate dehydrogenase (GAPDH)and heat shock protein60(HSP60)in the cytosol and mitochondria(Supplementary Figure S3online).Immunoblot analysis
Rat and mouse kidney tissue,and RPTCs were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes.Membranes were incubated with primary antibodies to calpains1,2,and10,and HSP60(used to normalize mitochondrial fractions),or GAPDH.The primary antibodies used were m-calpain(domain IV)(Calbiochem,La Jolla,CA;1:1000), m-calpain(domains III and IV)(Abcam,Cambridge,MA;1:1000), calpain10(Abcam;1:1000),caspase3(StressGene,San Diego,CA; 1:1000),HSP60(Abcam;1:1000),ATP Synthase b(1:1000),NDUFB8 (Invitrogen,Carlsbad,CA;1:1000),and GAPDH(Fitzgerald Anti-bodies,Acton,MA;1:1000).We previously reported that ND6was a substrate for calpain10.18The anti-ND6antibody(Invitrogen) manufacturer recently informed us that the antibody actually identi?es the mitochondrial complex1protein NDUFB8.Thus, NDUFB8is a substrate of mitochondrial calpain10.Antibody incubation was followed by a horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody(Santa Cruz,Santa Cruz, CA;1:1000).Immunoreactive protein was visualized by enhanced chemiluminescence(Amersham,GE Healthcare,Piscataway,NJ)and imaged using an Alpha Innotech imaging station(Santa Clara,CA). STZ diabetic model
Male Sprague–Dawley rats,8weeks of age(200–250g),were starved for 16h and injected once with STZ(55mg/kg,tail vein)in sodium citrate buffer.32–34Afterward,rats were given drinking water supplemented with sucrose(15g/l)for48h to limit mortality as stores of insulin are released from damaged pancreatic islets.Aged matched males rats were controls.After24h,diabetes was con?rmed in STZ-treated rats by measuring tail vein plasma glucose.At10weeks after diabetes induction,rats were killed and blood and kidneys were harvested. Ob/ob mice
For the ob/ob mice study,8-week-old male ob/ob(leptinà/à)and lean controls(Jackson Labs,Bar Harbor,Maine)were used.Mice were killed and blood and kidneys were harvested.Kidneys were homogenized and immunoblot analyses were performed. Reverse transcription PCR
Rat kidney samples were homogenized with TRIZOL reagent (Invitrogen)according to the manufacturer’s protocol to extract total RNA.The mRNA was subjected to reverse transcription to DNA using Moloney murine leukemia virus(MMLV)reverse transcriptase (Invitrogen)in the presence of oligo dT primers.PCR was performed using primers for calpains1,2,and10,which has previously been reported.15Ampli?cation of a-GAPDH was used as internal control for normalizing PCR ef?ciency.Products were electrophoresed on 1.5%agarose gel and stained with ethidium bromide.
Calpain activity
Calpain activity was assayed spectrophotometrically using the calpain substrate succinyl-Leu-Leu-V al-Tyr-7-amino-4-methylcoumarin (Bachem,T orrance,CA)as previously described.18
TUNEL assay
The In Situ Cell Death Detection kit(Roche,Indianapolis,IN)was used.After deparaf?nization,the sections were treated with proteinase K(20g/ml)and kit protocol was followed.TUNEL-positive cells were counted in10non-overlapping?elds in each section under?100magni?cation.
Measurement of RPTC oxygen consumption(QO2)
QO2was monitored as previously described using a Clark-type oxygen electrode.18After the basal rate of QO2was determined, carbonyl cyanide-p-tri?uoromethoxyphenylhydrazone(?nal con-centration?10m mol/l)was injected to obtain uncoupled QO2. Assessment of nuclear morphology
Nuclear morphology was assessed as previously described.35Visua-lization of4,6-diamidino-2-phenylindole staining was performed using a?uorescence microscope and condensed nuclei in12cells from10high-powered?elds were counted for each treatment group. In vivo siRNA
In vivo siRNA was administered as previously described.26Male Sprague–Dawley rats,6–8weeks of age,were treated with calpain 10siRNA(50-CCAGGACAUUUGUGCCACACCUCAA-30)(Invitro-gen)or a negative control(scramble)(Invitrogen),with a phospho-rothioate backbone that decreases degradation and extends its effectiveness,in a sterile siRNA resuspension buffer(Invitrogen)to decrease renal calpain10.Rats were treated with20nmol of siRNA directed against calpain10or scrambled siRNA by tail vein injection.Tissues were isolated,homogenized,and immunoblot performed.Serum creatinine measurements were performed with the appropriate kit(Bioassay Systems,Hayward,CA).
Statistical analysis
RPTCs isolated from one rabbit represents one experiment(n?1). The appropriate analysis of variance was performed for each data set using SigmaStat statistical software(Ashburn,VA).Individual means were compared with Fisher’s protected least signi?cant difference test with P p0.05being considered statistically signi?cant. DISCLOSURE
All the authors declared no competing interests. ACKNOWLEDGMENTS
This research was supported by NIEHS grant ES-012239(to RGS)and NIGMS grant GM-084147(to RGS),and by the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs.MDC was supported by the National Institutes of Health Training grant T32HL007260.Animal facilities were funded by NIH grant C06RR-015455.
Disclaimer
The article content does not represent the views of the Department of Veterans Affairs of the US Government.
SUPPLEMENTARY MATERIAL
Figure S1.The effect of11mM glucose and11mM D-mannitol on cytosolic and mitochondrial calpain10.
Figure S2.The effect of siRNA on calpain protein in rat kidney and liver.
Figure S3.Relative purity of RPTC mitochondrial and cytosolic fractions.
Supplementary material is linked to the online version of the paper at https://www.360docs.net/doc/6512369707.html,/ki
REFERENCES
1.Jerums G,Premaratne E,Panagiotopoulos S et al.New and old markers of
progression of diabetic nephropathy.Diabetes Res Clin Pract2008;
82(Suppl1):S30–S37.
2.Dalla Vestra M,Saller A,Bortoloso E et al.Structural involvement in type1
and type2diabetic nephropathy.Diabetes Metab2000;26(Suppl4):8–14.
3.Futrakul N,Futrakul P.Renal microvascular and tubular injuries in type II
diabetic nephropathy.Kidney Int2008;74:390;author reply390–1.
4.Gilbert RE,Wu LL,Kelly DJ et al.Pathological expression of renin and
angiotensin II in the renal tubule after subtotal nephrectomy.
Implications for the pathogenesis of tubulointerstitial fibrosis.
Am J Pathol1999;155:429–440.
5.Hall JC,Sordahl LA,Stefko PL.The effect of insulin on oxidative
phosphorylation in normal and diabetic mitochondria.J Biol Chem1960;
235:1536–1539.
6.Paul DS,Harmon AW,Winston CP et al.Calpain facilitates GLUT4vesicle
translocation during insulin-stimulated glucose uptake in adipocytes.
Biochem J2003;376:625–632.
7.Tomita M,Mukae S,Geshi E et al.Mitochondrial respiratory impairment
in streptozotocin-induced diabetic rat heart.Jpn Circ J1996;60:
673–682.
8.Oliveira PJ,Esteves TC,Seica R et al.Calcium-dependent mitochondrial
permeability transition is augmented in the kidney of Goto-Kakizaki
diabetic rat.Diabetes Metab Res Rev2004;20:131–136.
9.Goll DE,Thompson VF,Li H et al.The calpain system.Physiol Rev2003;83:
731–801.
10.Huang Y,Wang KK.The calpain family and human disease.Trends Mol
Med2001;7:355–362.
11.Hanis CL,Boerwinkle E,Chakraborty R et al.A genome-wide search for
human non-insulin-dependent(type2)diabetes genes reveals a major susceptibility locus on chromosome2.Nat Genet1996;13:161–166. 12.Harris F,Biswas S,Singh J et al.Calpains and their multiple roles in
diabetes mellitus.Ann NY Acad Sci2006;1084:452–480.
13.Lynn S,Evans JC,White C et al.Variation in the calpain-10gene affects
blood glucose levels in the British population.Diabetes2002;51:247–250.
14.Cushman SW,Wardzala LJ.Potential mechanism of insulin action on
glucose transport in the isolated rat adipose cell.Apparent translocation of intracellular transport systems to the plasma membrane.J Biol Chem 1980;255:4758–4762.
15.Otani K,Han DH,Ford EL et al.Calpain system regulates muscle mass and
glucose transporter GLUT4turnover.J Biol Chem2004;279:20915–20920.
16.Marshall C,Hitman GA,Partridge CJ et al.Evidence that an isoform of
calpain-10is a regulator of exocytosis in pancreatic beta cells.Mol
Endocrinol2005;19:213–224.
17.Sreenan SK,Zhou YP,Otani K et al.Calpains play a role in insulin secretion
and action.Diabetes2001;50:2013–2020.
18.Arrington DD,Van Vleet TR,Schnellmann RG.Calpain10:a mitochondrial
calpain and its role in calcium-induced mitochondrial dysfunction.Am J Physiol Cell Physiol2006;291:C1159–C1171.
19.Giguere CJ,Covington MD,Schnellmann RG.Mitochondrial calpain10
activity and expression in the kidney of multiple species.Biochem Biophys Res Commun2008;366:258–262.
20.Covington MD,Arrington DD,Schnellmann RG.Calpain10is required for
cell viability and is decreased in the aging kidney.Am J Physiol Renal
Physiol2009;296:F478–F486.
21.Turner MD,Fulcher FK,Jones CV et al.Calpain facilitates actin
reorganization during glucose-stimulated insulin secretion.Biochem
Biophys Res Commun2007;352:650–655.
22.Mukai H,Kawakami S,Hashida M.Renal press-mediated transfection
method for plasmid DNA and siRNA to the kidney.Biochem Biophys Res Commun2008;372:383–387.
23.Takabatake Y,Isaka Y,Mizui M et al.Chemically modified siRNA
prolonged RNA interference in renal disease.Biochem Biophys Res
Commun2007;363:432–437.
24.van de Water FM,Boerman OC,Wouterse AC et al.Intravenously
administered short interfering RNA accumulates in the kidney and
selectively suppresses gene function in renal proximal tubules.Drug
Metab Dispos2006;34:1393–1397.
25.Wolfrum C,Shi S,Jayaprakash KN et al.Mechanisms and optimization of
in vivo delivery of lipophilic siRNAs.Nat Biotechnol2007;25:1149–1157.
26.Molitoris BA,Dagher PC,Sandoval RM et al.siRNA targeted to p53
attenuates ischemic and cisplatin-induced acute kidney injury.J Am Soc Nephrol2009;20:1754–1764.
27.Diaz-Villasenor A,Hiriart M,Cebrian ME et al.The activity of calpains in
lymphocytes is glucose-dependent and is decreased in diabetic patients.
Blood Cells Mol Dis2008;40:414–419.
28.Li Y,Feng Q,Arnold M et al.Calpain activation contributes to
hyperglycaemia-induced apoptosis in cardiomyocytes.Cardiovasc Res
2009;84:100–110.
29.Harwood SM,Allen DA,Raftery MJ et al.High glucose initiates calpain-
induced necrosis before apoptosis in LLC-PK1cells.Kidney Int2007;71: 655–663.
30.Nowak G,Schnellmann RG.Improved culture conditions stimulate
gluconeogenesis in primary cultures of renal proximal tubule cells.
Am J Physiol1995;268:C1053–C1061.
31.Nowak G,Schnellmann RG.L-ascorbic acid regulates growth and
metabolism of renal cells:improvements in cell culture.Am J Physiol
1996;271:C2072–C2080.
32.Frier BC,Noble EG,Locke M.Diabetes-induced atrophy is associated with
a muscle-specific alteration in NF-kappaB activation and expression.
Cell Stress Chaperones2008;13:287–296.33.Gulen S,Dincer S.Effects of leptin on oxidative stress in healthy and
Streptozotocin-induced diabetic rats.Mol Cell Biochem2007;302:
59–65.
34.Tobin BW,Lewis JT,Chen DZ et al.Insulin secretory function in relation to
transplanted islet mass in STZ-induced diabetic rats.Diabetes1993;42: 98–105.
35.Cummings BS,Kinsey GR,Bolchoz LJ et al.Identification of caspase
independent apoptosis in epithelial and cancer cells.J Pharmacol Exp Ther2004;310:126–134.
硫酸氨基葡萄糖胶囊
罗非昔布片 【药品名称】 通用名称:罗非昔布片 英文名称:Rofecoxib Tablets 【成份】 本品主要成分为罗非昔布,其化学名称为4-[4-(思虑磺酰基)苯基]-3-苯基-2(5H)呋喃酮,为一特异环氧化酶-2(COX-2)抑制剂。 【适应症】 1.骨关节炎症状和体征的短期和长期治疗。 2.缓解疼痛。 3.治疗原发性痛经。 【用法用量】 口服给药。骨关节炎:推荐起始剂量为12.5mg ,一日一次,一些患者剂量增加至此25mg,一日一次,可能取得更好的疗效,最大推荐剂量为每日25mg。缓解急性疼痛和治疗原发性痛经:推荐首次剂量为50mg,一日一次。随后剂量为25至50mg。最大推荐剂量为每日50mg。可连续服用5天。老年患者。轻到中度肾功能不全患者(肌酐清除率为30-80ml/min)和轻到中度肝功能不全患者(Child-Pugh评为5-9)不需用调整剂量。无严重肝功能不全患者(Child-Pug 评分为5-9)不需调整剂量。无严重肝功能不全患者(Child-Pugh值大于9)的临床数据。万络TM可与食物同服或单独服用。 【不良反应】 在临床试验中,对大约会5400例使用万络TM患者进行了安全性评价,其中近800例患者服药1年或更长时间。以下是在临床研究中患者用药达6个月所报告的与药物有关的不良反应。这些不良反应发生在≥2%的服用万络TM的患者中。其发生率较安慰剂组高:下肢水肿、高血压、心口灼热、消化不良、上腹不适、恶心、腹泻。另外罕见有口腔溃肠的报告。服用万络TM1
年或更长时间,不良反应与上述报告相似。 【禁忌】 万络TM禁用于对本产品任一成分过敏的患者。 【注意事项】 与其它已知前列腺素合成抑制剂一样,应避免在妊娠后期使用万络TM。因为它可导致动脉管的提前闭合.晚期肾脏疾病患者不建议使用万络TM治疗。严重脱水患者须慎重使用万络TM。建议在开始使用万络TM治疗前进行补液。对已有水肿和心功能不全的患者给予万络TM时,应考虑到可能导致体液潴留和水肿。如存在持续性肝功能检查异常(三倍于正常值上限),应停用万络TM。曾因水杨酸盐或非选择性环氧化酶抑制剂而导致急性哮喘发作、荨麻疹或鼻炎加重的患者应慎重用万络TM.这些反应的病理生理学尚不清楚,医生应权衡给予万络TM可能的效果和危险性.万络TM可掩盖感染的症状一发烧.当给正在接受抗感染治疗的患者服用万络TM时,医生应该意识这一点. 【药理作用】 环氧酶(COX)的2个异构体COX-1和COX-2催化人体前列腺素的合成。COX-1是正常的细胞组成蛋白,在正常组织中表达,维持体内前列腺素的生理功能,包括胃肠粘膜的保护功能。COX-2是炎症时COX的诱导形式,主要在炎症细胞中表达,产生介导炎症和疼痛的前列腺素。NSAIDs对炎症的有效治疗作用源于其对COX-2的抑制。而胃肠道穿孔、溃疡、出血等不良反应则归于对COX-1的抑制。 本品为口服有效的选择性COX-2抑制剂。每日1次服用足以保持有效的作用,疗效与剂量有关。体内外研究表明,使用10倍治疗剂量时,未见本品对COX-1有抑制作用,也不会抑制胃前列腺素的合成,且对出血时间无影响。临床评价: 1.784例患有膝或髋骨关节炎患者随机分为3组,分别服用:罗非昔地1 2.5mg,qd,罗非
2019年执业药师继续教育 创新机制–肾脏钠葡萄糖共转运蛋白2 SGLT-2抑制剂-达格列净...考试
创新机制–肾脏钠葡萄糖共转运蛋白2 SGLT-2抑制剂-达格列净...考试 返回上一级 单选题(共10 题,每题10 分) 1 . 最近统计中国糖尿病患病率在成人中达到() ? A.11.6% ? B.9.8% ? C.6.6% ? D.4.5% 我的答案: A 参考答案:A 答案解析:暂无 2 . 由于人口的原因世界上患糖尿病人数最多的国家是() ? A.美国 ? B.中国 ? C.印度 ? D.日本 我的答案: B 参考答案:B 答案解析:暂无 3 . 肾脏葡萄糖转运:SGLT2负责()肾脏葡萄糖的重吸收 ? A.90% ? B.80% ? C.70% ? D.60% 我的答案: A 参考答案:A 答案解析:暂无 4 . 糖尿病是代谢综合征的一部分表现,型糖尿病患者合并高血压和(或)脂代谢紊乱的达到() ? A.70% ? B.68% ? C.60% ? D.55% 我的答案: C 参考答案:C 答案解析:暂无 5 . 第一个SGLT-2抑制剂来源于() ? A.草根 ? B.苹果树皮 ? C.苹果树叶 ? D.以上都是
我的答案: B 参考答案:B 答案解析:暂无 6 . SGLT2抑制剂肾脏保护的间接获益包括() ? A.改善血糖控制 ? B.降低血压 ? C.降低体重 ? D.以上都是 我的答案: D 参考答案:D 答案解析:暂无 7 . 与二甲双胍联合用药长期控制血糖非常好的药物是() ? A.安慰剂 ? B.二甲双胍 ? C.达格列净 ? D.都可以 我的答案: C 参考答案:C 答案解析:暂无 8 . 达格列净减重作用主要源于() ? A.体内脂肪减少 ? B.体内肌肉的减少 ? C.体内脂肪增加 ? D.体内脂肪增加 我的答案: A 参考答案:A 答案解析:暂无 9 . 达格列净初始单药治疗低血糖风险与安慰剂相比() ? A.相当 ? B.略高 ? C.高出很多 ? D.以上都对 我的答案: A 参考答案:A 答案解析:暂无 10 . 达格列净多重获益优势为糖尿病综合管理带来新的希望包括()? A.不增加心血管事件风险 ? B.减少肾病风险 ? C.快速、强效、持久的血糖控制,低血糖风险低 ? D.以上都是 我的答案: D 参考答案:D 答案解析:暂无
氨基葡萄糖盐酸盐与氨基葡萄糖盐酸盐的区别
氨基葡萄糖盐酸盐与氨基葡萄糖盐酸盐的区别 氨基葡萄糖硫酸盐治疗髋关节骨关节炎 荷兰鹿特丹Erasmus医学中心Rozendaal等报告,氨基葡萄糖硫酸盐治疗髋关节骨关节炎无效,在减轻髋关节骨关节炎症状和延缓疾病进展方面,其效果与安慰剂相比并无优势[Ann Intern Med 2008,148(4): 268]。 该研究是一项随机对照试验,纳入222例髋关节骨关节炎患者。纳入者每天口服 氨基葡萄糖硫酸盐1500 mg或安慰剂治疗,持续2年。评估氨基葡萄糖硫酸盐治 疗髋关节骨关节炎的效果。 主要观察指标为治疗24个月过程中大略和McMaster大学(WOMAC)疼痛和功能评分与治疗24个月后关节间隙狭窄情况。次要观察指标为在治疗3、12、24个月后 WOMAC 疼痛、功能和僵硬程度评分。在治疗前,两组患者一般情况和临床指标无显着差异。 结果显示,总体上两组患者的WOMAC疼痛评分和WOMAC功能评分无显着差异。治疗24个月后,两组患者的关节间隙狭窄也无显着差异。只有在一项基于对缺失评估数据(因为患者接受了全髋关节置换手术)进行的最大限度假设的敏感性分析 中得出支持氨基葡萄糖硫酸盐无效的结果。 研究者指出,该研究的局限性在于在研究过程中有20例患者接受了全髋关节置 换手术,这可能影响分析的结果。 氨糖,又称为氨基葡萄糖,葡萄糖胺,市面上主要有两类,一类是氨基葡萄糖盐酸盐,一类是氨基葡萄糖硫酸盐. 氨基葡萄糖盐酸盐:英文D-Glucosamine Hydrochloride,分子式C6H13NO5·HCl,分子量,白色结晶,无气味,略有甜味,易溶于水,微溶于甲 醇,不溶于乙醇等有机溶剂,它对人体具有重要的生理功能,参与肝肾解毒,发挥抗炎护肝作用,对治疗风湿性关节炎症和胃溃疡有良好的疗效,是合成抗生素和抗癌药物的主要原料,还可应用于食品,化妆品和饲料添加剂 中.氨基葡萄糖盐酸盐是由天然的甲壳质提取的,是一种海洋生物制剂,是硫酸软骨素的主要成分.它能促进人体粘多糖的合成,提高关节滑液的粘 性,能改善关节软骨的代谢,有利于关节软骨的修复,具有明显的消炎镇痛 作用.它具有促进抗生素注射效能的作用,可供糖尿病者作营养补助剂. 氨基葡萄糖硫酸盐:产品英文名称D-Glucosamine sulfate 别名:D-氨基葡萄糖硫酸盐;D-氨基葡萄糖硫酸钾盐 CAS 号 29031-19-4 分子式分子量产品英文名称 D-Glucosamine sulfate 氨基葡萄糖硫酸 钠盐白色结晶粉末,无气味,略有甜味,易溶于水,微溶于甲醇,不溶于乙醇 等有机溶剂. 用途: 制药原料.对风湿性关节炎,心脏病,肺炎及骨折均有 辅助治疗作用,另有吸收自由基,抗衰老,减肥,调节内分泌等多种有益的 生理作用. 在关节炎治疗效果来说,氨基葡萄糖盐酸盐比硫酸盐效果好,由于其盐酸盐不含钠离子,副作用小.纯度更纯,分子更小,人体容易吸收,可以直
胰岛素调控葡萄糖转运蛋白4转位的研究进展_于海佳
DOI:10.3969/cmba.j.issn.1673-713X.2015.01.011· 综述·胰岛素调控葡萄糖转运蛋白4转位的 研究进展 于海佳 胰岛素抵抗和糖代谢异常是 II 型糖尿病的主要病理特征。机体在正常情况下通过胰岛素等相关激素能够非常精准地调控血液中的葡萄糖。伴随着能量摄入,升高的血糖水平会刺激胰岛β细胞分泌胰岛素。血液中过量的葡萄糖被快速地转运至细胞内,从而使机体维持正常的血糖水平。胰岛素调控葡萄糖摄取主要是通过葡萄糖转运蛋白4(glucose transporter 4,GLUT4)从细胞内转位到质膜上来实现的。有关胰岛素是如何介导 GLUT4 转位和葡萄糖摄取的研究对于治疗糖尿病和发展疾病早期诊断方法具有重要的意义。本文综述了近年来在胰岛素信号调控下 GLUT4 转位方面的相关研究进展。 1 GLUT4 与糖稳态调控 GLUT4 是由 SLC2A4 基因编码的糖转运蛋白,能够以不依赖于 ATP、协助运输的方式运送葡萄糖穿过细胞质膜。GLUT4 具有 12 次跨膜蛋白结构域,广泛分布于骨骼肌和脂肪组织等胰岛素响应性组织中[1-2]。除了 GLUT4 外,这些组织还表达其他的一些糖转运蛋白,例如 GLUT1。与其他糖转运蛋白不同的是,GLUT4 在细胞内的分布受到胰岛素的调控。GLUT1 等其他糖转运蛋白主要在基础状态(血糖水平低)下介导细胞对葡萄糖的摄取,而 GLUT4 在基础状态主要存在于胞内的各种膜结构中,只有少于 5% 的 GLUT4 位于细胞膜上。当机体进食后血糖水平快速升高,葡萄糖会促进胰岛素分泌增加。胰岛素促使 GLUT4 从胞内膜结构转移到细胞膜表面上,细胞表面上的 GLUT4 浓度在胰岛素的刺激下可以增加到其在基础状态时的 5 ~ 30 倍[3]。GLUT4 通过摄取和清除血液中的葡萄糖来维持血糖平衡。当胰岛素浓度降低时,GLUT4 通过胞吞作用回到细胞内,细胞表面的 GLUT4 重新恢复到基础状态时的水平。 GLUT4 在机体糖稳态调控过程中发挥着重要作用,在II 型糖尿病患者的脂肪组织中,GLUT4 在 mRNA 和蛋白质表达水平上都有明显减少[4]。在小鼠模型中,GLUT4 蛋白表达水平降低使小鼠产生胰岛素抵抗和糖尿病[5]。GLUT4 在肌肉组织和脂肪组织中过量表达可以改善小鼠的血糖控制和糖耐受不良[6-7]。在细胞水平上,肌肉组织和脂肪组织中减少 GLUT4 的表达会引起肌肉细胞和脂肪细胞对葡萄糖的摄取减少并产生胰岛素抵抗[8]。2 胰岛素调控 GLUT4 转位的信号通路 对于胰岛素调控骨骼肌和脂肪组织的葡萄糖摄取,目前研究者们认为主要是通过磷酸肌醇 3 激酶(PI3K)信号通路来实现的(图1)。胰岛素从胰岛β细胞分泌后,首先结合细胞表面上的跨膜胰岛素受体(IR)并激活胰岛素受体酪氨酸激酶。这会促使胰岛素受体底物蛋白(IRS)酪氨酸磷酸化,激活 PI3K。PI3K 与二磷酸肌醇(PIP2)发生作用,使 PIP2 转化为三磷酸肌醇(PIP3)[9]。PIP3 的水平升高激活了含有 PH 结构域的丝氨酸/苏氨酸激酶 PDK1 和mTORC2,并随后激活蛋白激酶 AKT。 AKT 有 3 个异构体,但是只有 AKT2 在胰岛素刺激GLUT4 转运过程中起关键作用。George 等[10]报道在胰岛素抵抗和糖尿病中发现了 AKT2 突变。AS160(又称为TBC1D4,分子量 160 kD)是 AKT2 的一个重要底物,在脂肪和肌肉组织中过量表达 AS160 磷酸化位点突变体能抑制胰岛素依赖的 GLUT4 转位和葡萄糖摄取,敲除 AS160 和其类似功能蛋白 TBC1D1,可显著减少胰岛素刺激的葡萄糖运输[11]。一份最新的报道发现格陵兰人近年来持续升高的 II 型糖尿病发生率正是由于 AS160 发生了突变。研究人员证实了在 2575 个调查个体中有 17% 的 AS160 等位基因存在 p.Arg684Ter 突变,同时伴随有胰岛素抵抗和血糖升高[12]。AS160 含有一个 GTP 酶激活蛋白(GAP)结构域,其能特异地作用于 G 蛋白 Rab。Rab 是一类能促进囊泡运输的 GTP 结合蛋白,通过与 GDP 结合的失活状态向其活化状态转化来催化膜运输。作为一个负调控因子,AS160 在基础状态下处于去磷酸化状态,能通过 GTP 酶将 GTP 转化成 GDP。这使 Rab 蛋白处于失活状态,从而抑制了 GLUT4 囊泡在细胞内的运输。在胰岛素刺激下,AS160 的五个氨基酸残基 Ser318、Ser570、Ser588、Thr642 和 Ser751 被 AKT2 磷酸化而丧失了 GAP 活性[13],使Rab 蛋白可以与 GTP 结合,促进 GLUT4 囊泡运输和GLUT4 的膜转位。在基础状态下的脂肪细胞中敲低 AS160 的表达,会使部分 GLUT4 囊泡运输至细胞表面,从而增加了细胞表面的 GLUT4 水平[14]。Rab10 是 AS160 一个重要下游结合 Rab 蛋白。在脂肪细胞中敲低 Rab10 的表达会抑制胰岛素引起的 GLUT4 转位。在敲低 AS160 的同 作者单位:80309 美国,科罗拉多大学博尔德分校分子细胞发育生物学系,Email:haijia@https://www.360docs.net/doc/6512369707.html, 收稿日期:2014-08-18
对葡萄糖转运蛋白的讨论
对葡萄糖转运蛋白的讨论 关键词:葡萄糖转运蛋白糖尿病胰岛素释放障碍胰岛素抵抗 葡萄糖转运蛋白是细胞转运葡萄糖的 载体。研究发现,葡萄糖转运蛋白是一个蛋白家族,包括多种蛋白,它们在体内的公布以及与葡萄糖分子的亲合力差异显着。其中GLUT2和GLUT4尤为重要。GLUT2是胰岛B 细胞膜上的转运蛋白,在血糖浓度升高时,促进GLUT2对葡萄糖的转运功能,继而刺激胰岛素释放。GLUT4在脂肪细胞和肌细胞中表达,胰岛素刺激GLUT4在脂肪细胞和肌细胞或表达,胰岛素刺激GLUT4分子转移到细胞膜上,促进葡萄糖分子的转运过程。GLUT2和GLUT4分子的研究对于糖尿病的胰岛素释放障碍和胰岛素抵抗有重要意义。 1GLUT的分类 除了肾和肠道有能量依赖性的钠-葡萄糖协同转运外,其它大多数细胞都有非能量依赖的转运体存在。它们将葡萄糖分子从高
浓度向低浓度载过细胞膜。现已发现至少存在五种这样的转运蛋白,它们对葡萄糖的转运有各自不同的特点,分为GLUT1、GLUT2、GLUT3、GLUT4和GLUT5。 GLUT1分子在人类所有组织中均存在, 它调节葡萄糖摄取。它对葡萄糖分子有很高的亲合力,因此在相对低浓度葡萄糖的状态下也能转运葡萄糖分子。由于这个原因,GLUT1是一种重要的脑血管系统成分,保证 足够血浆葡萄糖分子转运进入中枢神经系统。 与GLUT1不同,GLUT2分子对葡萄糖亲合力极低,似乎仅在血浆葡萄糖水平相对较高时才作为转运体发挥载体功能。例如饭后,胰岛B细胞和肝细胞中起葡萄糖转运功能的分子就是GLUT2。这种生理功能抑制了正常状态或饥饿条件下肝脏对葡萄糖分子的摄 取和胰岛素不正常分泌。OgawaY等人研究发现,对于Ⅱ型、Ⅰ型早期糖尿病人和胰腺移植失败的病人,在血糖浓度升高时,普通B 细胞中GLUT2分子的表达有所下降。因此他们得出结论:对于上述病人,高血糖通过对
葡萄糖转运蛋白与肺癌
!!作者单位" #,"""#杭州#浙江大学医学院附属第一医院呼吸科葡萄糖转运蛋白与肺癌 钟秀君!周建英 !!肿瘤细胞无法调控的增殖是肿瘤细胞最主要特征#而细胞数的增多导致细胞耗氧量不断增加#造成肿瘤缺氧#这在人实体瘤中表现尤其明显’肿瘤在适应缺氧时#葡萄糖摄入增多以提供所需的能量#此方式通过葡萄糖转运蛋白%@?I 9<;237/:;T <7327#[?I 3&合成增加来实现’[?I 3是介导细胞葡萄糖摄取的主要载体#与正常细胞$组织及良性病变相比#恶性肿瘤细胞对葡萄糖的代谢率增加’而糖代谢的增高与[?I 3及基因的异常表达有关’本文就[?I 3及其同肿瘤的关系作一综述’ !!H ;<9的分类和在组织中的分布细胞不能通过简单的弥散方式吸收葡萄糖#它必须借助一种特殊蛋白质#即葡萄糖转运蛋白’由于不同组织对葡萄糖需求不同#故可能有不同的葡萄糖转运蛋白’目前用基因探针方法# 已发现了’种不同的葡萄糖转运蛋白%[?I 3,\-$[?I 3*\0& ’[?I 3,在人类所有组织中均存在#它对葡萄糖具有很高的亲和力#可调节葡萄糖摄取’[?I 3!出现在能释放葡萄糖入血的器官中#如肠$肝$肾$及胰腺的/细胞#对葡萄糖亲和力极低#似乎仅在血浆葡萄糖水平相对较高时才作为转运体发挥载体功能’[?I 3#在脑神经元中被发现# 存在于人类所有组织中’对葡萄糖分子也有高亲和性’[?I 3(是肌肉和脂肪细胞主要的转运蛋白# 一般情况下#不能起转运葡萄糖的作用#仅在胰岛素的信号刺激下#能促进饭后葡萄糖进入上述组织中储存起来’[?I 3-主要存在于小肠及肾脏#主要作为果糖转运体’[?I 3.基因是一个假基因#不在蛋白水平表达’[?I 3*是肝微粒体[?I 3#与[?I 3!有.’)序列一致性’[?I 3’是主要表达于睾丸及受胰岛素调控的组织中’[?I 30在脾$外周白细胞$脑组织中表达’这’种葡萄糖转运蛋白转运葡萄糖都是按浓度梯度进行的’还有一种是钠离子依赖的协同转运蛋白%$[&H &#它逆浓度主动转运葡萄糖#是耗能过程#有$[&H ,%在小肠中表达明显#肾$肝$肺中少量表达&和$[&H !%肾中表达高#小肠中少&两种’ -!H ;<9与肿瘤 -"!![?I 3表达与肿瘤的生物学行为!各种葡萄糖转运蛋白在不同类型肿瘤中作用可能各不相同#[?I 3,可能是大多数肿瘤中表达的主要角色’其在 各部位肿瘤中表达(, )大致如下’头颈部"见于基底上皮细胞癌和口腔癌*胰腺"和G Q [%!\脱氧氟代\Q \葡萄糖&表达正相关*结肠"增强的表达与不良的预后有关*阴茎"在增生的病变处表达增强*胃食道"胃中高度表达#与M /77233食管有关*肾$膀胱"高度表达但与肿瘤分级无关*甲状腺"仅在恶性肿瘤中表达*肺"仅在恶性肿瘤中表达#在肿瘤中心表达更高#是非小细胞肺癌的预兆*乳腺"过度表达但与肿瘤大小$受体$淋巴结状态无关*脑"[?I 3,比[?I 3#表达低#且与星形细胞瘤分级相关*卵巢"过度表达#且与 肿瘤分级有关*皮肤"表达提示增生性病变’国外( !)亦有报道[?I 3,在肺癌$结直肠癌$乳腺癌等多种肿瘤中均有过度表达#而且其表达水平与肺癌及结直肠癌的临床分期$ 转移和预后密切相关’-"!"!![?I 3表达与癌发生的关系!在一些恶性肿 瘤中[?I 3表达与癌的形成无关#如在胃癌(# )中用免疫组织化学方法检测发现胃腺瘤$ 癌前病变$早期胃癌中检测不到[?I 3,表达#而只在易浸润$发生转移的胃癌中检测到#[?I 3,表达并不随着胃癌的发展而 逐渐增高’而对胆囊癌(()的免疫组织化学实验发 现#[?I 3,的表达与胆囊癌的形成及进展高度相关’-"!"-![?I 3异常表达与癌分化程度的关系! Y 对葡萄糖转运蛋白的讨论
关键词:葡萄糖转运蛋白糖尿病胰岛素释放障碍胰岛素抵抗葡萄糖转运蛋白是细胞转运葡萄糖的载体。研究发现,葡萄糖转运蛋白(后简称GLUT)是一个蛋白家族,包括多种蛋白,它们在体内的公布以及与葡萄糖分子的亲合力差异显著。其中GLUT2和GLUT4尤为重要。GLUT2是胰岛B细胞膜上的转运蛋白,在血糖浓度升高时,促进GLUT2对葡萄糖的转运功能,继而刺激胰岛素释放。GLUT4在脂肪细胞和肌细胞中表达,胰岛素刺激GLUT4在脂肪细胞和肌细胞或表达,胰岛素刺激GLUT4分子转移到细胞膜上,促进葡萄糖分子的转运过程。GLUT2和GLUT4分子的研究对于糖尿病的胰岛素释放障碍和胰岛素抵抗有重要意义。1GLUT的分类除了肾和肠道有能量依赖性的钠-葡萄糖协同转运外,其它大多数细胞都有非能量依赖的转运体存在。它们将葡萄糖分子从高浓度向低浓度载过细胞膜。现已发现至少存在五种这样的转运蛋白,它们对葡萄糖的转运有各自不同的特点,分为GLUT1、GLUT2、GLUT3、GLUT4和GLUT5。GLUT1分子在人类所有组织中均存在,它调节葡萄糖摄取。它对葡萄糖分子有很高的亲合力,因此在相对低浓度葡萄糖的状态下也能转运葡萄糖分子。由于这个原因,GLUT1是一种重要的脑血管系统成分,保证足够血浆葡萄糖分子转运进入中枢神经系统。与GLUT1不同,GLUT2分子对葡萄糖亲合力极低,似乎仅在血浆葡萄糖水平相对较高时才作为转运体发挥载体功能。例如饭后,胰岛B细胞和肝细胞中起葡萄糖转运功能的分子就是GLUT2。这种生理功能抑制了正常状态或饥饿条件下肝脏对葡萄糖分子的摄取和胰岛素不正常分泌。OgawaY等人研究发现,对于Ⅱ型、Ⅰ型早期糖尿病人和胰腺移植失败的病人,在血糖浓度升高时,普通B细胞中GLUT2分子的表达有所下降。因此他们得出结论:对于上述病人,高血糖通过对GLUT2的下调作用减少葡萄糖诱导的胰岛分泌,加重病情。虽然,GLUT2分子是葡萄糖刺激胰岛素分泌的一个关键因子,但其他环节如糖激酶异常,ADP-核糖生成障碍等均与胰岛素分泌障碍有关,因此上述实验只能说明GLUT2分子在胰岛B细胞的葡萄糖转运中起着重要作用,其它结论还有待研究。GLUT3分子在所有组织中均已发现,主要作为神经元表面的葡萄糖转运体,它对葡萄糖分子也有高亲合性,负责将葡萄糖从脑脊液转运至神经元细胞。GLUT4主要存在于骨骼肌、脂肪细胞的胞浆中,一般情况下,不能起转运葡萄糖的作用,仅在胰岛素的信号刺激下,才能通过易位作用转运到细胞膜上,促进饭后葡萄进入上述组织中储存起来。GLUT5在人类小肠刷状缘上表达,主要作为果糖转运体,在肝脏也高度表达。2GLUT4分子是研究的一个热点糖尿病的发病机制归纳而言无外乎两个方面,一是胰岛素分泌不足,二是胰岛素抵抗。胰岛素抵抗的结果,血浆中胰岛素水平虽高,但血糖浓度还是比正常情况高。葡萄糖转运机制障碍是胰岛素抵抗的一个重要方面,也是现今研究的一个热点。在骨骼肌和脂肪细胞,胰岛素刺激葡萄糖转运过程如下:首先胰岛素与细胞膜上的受体结合,然后通过至今仍不明确的信号传递过程使含有GLUT4分子的囊泡从胞内池移动到细胞膜,然后与膜融合,将GLUT4分子固定在细胞膜上,从而发挥转运葡萄糖等C1-C3位置有相同结构的其它糖分子(如L-阿拉伯糖、D-木糖、半乳糖)的作用。 [!--empirenews.page--] 胰岛素抵抗虽然包括GLUT4转运活性的下降,但这种缺陷是否是GLUT4分子数量不足引起的呢?GarveywT等人研究证实,无论是在糖尿病人还是非糖尿病患者,只要存在胰岛素抵抗,GLUT4的数量并无明显减少,但GLUT4的易位作用发生了障碍,它们在高密度膜区异常积累,但不能转移到细胞膜上。这种现象在骨骼肌细胞和脂肪细胞中均已被发现。所以胰岛素抵抗的机制之一可能是GLUT4分子易位障碍,而不是合成、释放不足。既然GLUT4分子在葡萄糖转运过程中如此重要,它是如何发挥作用的呢?GLUT4分子镶嵌在细胞膜的脂质分子双层中,通过构象改变将葡萄糖分子运进细胞内,而不是借助蛋白本身的运动。即所谓的“ping pong”机制。这种构象改变可能与GLUT4分子的磷酸化、去磷酸化有关。JE-Reusch等人在脂肪细胞培养液中加入PTH,发现GLUT4磷酸化程度明显增加,而胰岛素刺激的去磷酸化作用显著降低。同时,PTH对GLUT4分子在细胞内分布没有影响。磷酸化的GLUT4分子在内在活性明显降低,可能与其构象改变障碍有
氨基葡萄糖硫酸盐报告
氨基葡萄糖硫酸盐报告 1概述 中文名称:氨基葡萄糖硫酸盐 英文文号:Glucosamine sulfate 分子式:C6H13NO5·H2SO4 结构式: 性状:白色结晶粉末,无气味,略有甜味。易溶于水,微溶于甲醇,不溶于乙醇等有机溶剂。 2 产品种类 氨基葡萄糖硫酸钠盐(Glucosamine Sulfate Sodium Chloride), 分子式:(C6H14NO5)2SO4·2NaCL 分子量:566.52 氨基葡萄糖硫酸钾盐(Glucosamine Sulfate Potassium Chloride)。 分子式:(C6H14NO5)2SO4·2KCL 分子量:605.52 3 生产工艺 目前目前已报道的工艺路线有酶法水解和化学水解两种。 本生产技术的原理是以甲壳素(N-乙酰氨基-2-脱氧-β-D-葡萄糖聚合物)为原料,经浓盐酸水解作用,使糖苷键断裂,酰胺键水解生成D-氨基葡萄糖盐酸盐,在和硫酸钠反应得到硫酸氨基葡萄糖复盐。
在生产工艺上,公司得到官能团保护法制氨基葡萄糖,即葡萄糖定向氨化制取氨基葡萄糖的专利转让消息。 其生产工艺流程如下: 4 氨基葡萄糖硫酸盐的用途 氨基葡萄糖硫酸盐主要用于医药,其它还可应用于食品,化妆品和饲料添加剂中,用途相当广泛。 在医药方面,参与肝肾解毒,发挥抗炎护肝作用,对治疗风湿性关节炎症和胃溃疡有良好的疗效,具有改善关节活动,缓解疼痛的作用。预防和治疗各种类型的骨性关节炎,如膝关节、髋关节、脊柱、肩、手、手腕和踝关节等部位的及全身性的骨性关节炎。 在食品工业方面,近年发现有吸收自由基、抗衰老、减肥、调节内分泌等多种有益的生理作用。可用氨基葡萄糖类保健食品。 在化工方面,是合成抗生素和抗癌药物的主要原料,亦可作为化妆品的营养性添加剂等。 5 市场分析 我国从上世纪70年代开始,利用沿海地区大量的海洋水产加工副产品——虾蟹壳生产甲壳素,进而开发出系列衍生产品,如壳聚糖和氨基葡萄糖等原料。国内氨基葡萄糖总产能为2800~3000吨,实际产量在1500吨左右。而国际市场上氨基葡萄糖原料的年消耗量在3000吨左右。大量原料以低价出口至欧美与日本。 目前我国60岁以上老人有1.3亿,其中至少40%患有不同程度的骨病,氨基葡萄糖作为一种在发达国家上市多年的骨保健产品,在国内无疑会有良好的发展前景,预期年销售峰值可达50亿~60亿元人民币。
硫酸氨基葡萄糖胶囊
硫酸氨基葡萄糖胶囊 【核准日期】 2007年10月4日 【修订日期】 2008年12月09日 【药品名称】 通用名:硫酸氨基葡萄糖胶囊 商品名:维固力 英文名:Glucosamine Sulfate Capsules 汉语拼音:Liu Suan An Ji Pu Tao Tang Jiao Nang 【成份】 本品主要成分为硫酸氨基葡萄糖 化学名称:2-氨基-2-脱氧-右旋-硫酸氨基葡萄糖·2氯化钠。(含氯化钠的硫酸氨基葡萄糖混合物,摩尔比为2:1) 化学结构式: 分子式:C12H28N2O14S·2NaCl 分子量:573.30 【性状】 本品为橙红色胶囊,内容物为白色至类白色粉末。 【适应症】 原发性或继发性骨关节炎。 【规格】 0.25g(以硫酸氨基葡萄糖计) 【用法用量】 口服,如果医师处方中没有特殊要求,建议每次2粒胶囊,每日3次(早晨及进餐时);连续用药6周,必要时可以6周以上,间隔2个月可重复使用。 【不良反应】 罕有轻度胃肠道不适,如恶心、便秘、腹胀和腹泻。有报道有些患者出现过敏反应,包括皮疹、瘙痒和皮肤红斑。 【禁忌】 对本品过敏的患者。 【注意事项】 未进行过对肝、肾功能不全患者的研究。本品的毒理学和药动学试验数据未显示出对这些患者的限制。但是,有严重肝、肾功能不全的患者应该在有医疗监护的条件下用药。 勿让儿童擅取。 【孕妇及哺乳期妇女用药】 在动物试验中,没有观察到本品对生殖功能和哺乳的不良影响。但由于缺乏在人体的研究,怀孕和哺乳期妇女应在权衡利弊后使用本品。怀孕头3个月应避免使用。 【儿童用药】 没有做过本品对儿童的有效性和安全性研究,因此没有关于儿童的推荐剂量。 【老年患者用药】
药物的修饰
有机药物的化学结构修饰 为提高药物的治疗效果,降低毒副作用,适应制剂要求,方便应用,可将药物化学结构进行修饰。修饰方法根据药物结构而定,近年来发展很快。 保持药物的基本结构,仅在某些功能基上作一定的化学结构改变,称为化学结构修饰。药物经化学修饰得到的化合物,在人体内又转化为原来的药物而发挥药效时,称原来的药物为母体药物(Parent Drug),修饰后的化合物为药物前体(Prodrug),亦称前体药物,简称前药。 第一节有机药物化学结构修饰的目的 药物化学结构修饰的目的在于:改善药物的转运与代谢过程,提高生物利用度;改善药物理化性质和不良嗅味;有利于药物与受体或酶的相互作用,引起相应的生物化学和生物物理的转变。 化学结构修饰的中心问题是:选择恰当的结构改变,使在生理条件下,能释放母体药物,并根据机体组织有酶、受体、pH等条件的差异,使母体药物释放有差异,而达到上述目的。大多数前药在体内主要经酶水解而释放母体药物。 一、使药物在特定部位作用: 一般情况下,药物的作用强度与其血浓度成正变关系。为提高药物的作用强度,就必须提高其血药浓度。将药物的结构进行修饰,成为无生物活性的前药,当药物前体运转到作用部位时,转化为母体药物,发挥其药效。这样,提高药物前体的血浓度,仅提高作用部位的母体药物浓度,使效力增加,而不显示副作用或较低。如癌细胞组织的特点是碱性磷酸酯酶、酰胺酶含量或活性高,pH值低。利用这些特点,设计了抗癌药的酯类和酰胺类前药。又如己烯雌酚二磷酸酯是治疗前列腺癌的效药物,服用后,到达癌细胞组织时,受酶分解为己烯雌酚,使癌细胞组织中的浓度高于正常细胞组织,有利于治疗,较少影响正常细胞。 二、提高药物的稳定性: 有的药物还原性较强,贮存过程中不稳定,易氧化分解失效。维生素C具烯二醇结构,还原性强,在存放过程中,极易受空气氧化失效。经修饰为苯甲酸维生素C酯,活性与维生素C相等,稳定性提高,其水溶液也相当稳定。 一些药物不经口服途径给药,疗效显著,但口服给药时,则效果不好。原因之一是这些药物对胃酸不稳定,被其分解失效。如羧苄青霉素口服效果差,其茚满酯则对胃酸稳定,可供口服,吸收性也改善。 三、改善药物的溶解性: 多种酸性或碱性有机药物或其盐类在水中溶度较低,溶解速度也较慢。将其制成适当的水溶性盐类,不仅溶度增大,溶解速度也相应提高,更能适应制剂要求。如苯妥英是一种弱酸性癫痫治疗药,一般是口服给药。癫痫发作时,需注射给药,但苯妥英水溶性低,其钠盐虽易溶于水,又碱性太强,易水解析出苯妥英使溶液混浊,而不适用于注射。将其分子引入N-磷酰氧甲基,作成磷酸3-羟基甲苯妥英酯(Phosphoric Acid 3-hydroxymenthyl phenytoin Ester,VI),其二钠盐的水溶性比苯妥英高4500倍,能满足注射要求。苯妥英开环形成的羧酸的氨基乙酸酯盐—苯妥英原(Prophenytoin,VII),水溶度大,在体内分解为脲基二苯乙酸,并自然环合成苯妥英而发挥作用。 四、改善药物的吸收性: 药物的吸收与脂水分配系数有关。如林可霉素的脂溶性差,脂水分配系数小,吸
硫酸氨基葡萄糖胶囊与盐酸氨基葡萄糖胶囊的区别
硫酸氨基葡萄糖胶囊和盐酸氨基葡萄糖胶囊的区别 一位老大妈步履蹒跚的来到药房问笔者:“孩子,有这个药没有?” 笔者接过大妈手中的药盒一看,原来是氨基葡萄糖胶囊,于是我很专业的从货架上取来一盒氨基葡萄糖胶囊,递到大妈手中,大妈端详了半天,告诉我说,这两个药不一样。 笔者以为,大妈口中的不一样指的是生产厂家不一样,正当我要给她解释的时候,她开口了:“医生说,硫酸氨基葡萄糖胶囊效果更好,盐酸的不比这个……”说着,便离开了药房。对于硫酸氨基葡萄糖胶囊和盐酸氨基葡萄糖胶囊,笔者都经营过,对它们的药理作用、治疗目的和治疗效果,也是有所了解。 但是这两个药,真的如大妈说的那样,有区别吗?如果有,它们的区别到底在哪里? 下面笔者和大家一起来学习这两个药的“庐山真面目”。众所周知,氨基葡萄糖胶囊是用来治疗骨关节炎的,骨关节炎的发病机制,是由于由于关节软骨蛋白多糖生物合成异常,导致关节退行性病变的一种疾病。 氨基葡萄糖是蛋白多糖合成的前体物质,是一种天然的氨基单糖,在它的作用下,软骨细胞可以产生更多正常多聚体结构的蛋白多糖,修复软骨细胞,抑制损伤软骨的各种酶的因素,防止损伤细胞的超氧化自由基的产生,促进软骨基质的
修复和重建,从而达到延缓骨关节炎病理过程和疾病进展的作用,改善关节活动,缓解症状。 临床上和其他药物(多用芬必得和甲钴胺)配合,主要用于用于治疗和预防全身所有部位的骨关节炎所带来的各种不适,缓解和消除疼痛、肿胀等症状、改善关节活动功能。而临床上之所以出现两种不同离子根的葡萄糖胶囊,并不是说那一个更好,而是近来的试验中,基于硫酸根的葡萄糖胶囊的数据比原来的基于盐酸根的数据要高。 具体一点就是说,实验数据上,硫酸氨基葡萄糖引用早一些,数据多一些,而相对盐酸派的来来说,实验数据少一些,引用的晚一些。但在临床使用中,两者的效果没有明显的差别。 这是因为骨关节炎是一种退行性病变,疾病的发生存在个体的差异,和患者的年龄、日常膳食中胶原蛋白的摄入程度、以及年轻时对关节的保护有关。 所以,大妈所说的硫酸氨基葡萄糖胶囊的效果大于盐酸氨基葡萄糖胶囊的说法,有一定的理论基础,但不完全符合。这可能就是这两个药在临床使用之中的最主要的区别。那么,是药三分毒,作为治疗骨关节炎的氨基葡萄糖胶囊,无论是哪个派别的,都不例外。1、过敏患者禁用,妊娠早期患者避免使用,哺乳期患者应在医生指导下使用;2、由于氨基葡萄糖是一种天然的产物,所以,无论是硫酸派还是盐酸哌,
钠-葡萄糖共转运蛋白2抑制剂对糖尿病肾病保护作用的研究进展
[15]TSUBAMOTO H ,KANAZAWA R ,INOUE K ,et al.Fertility ?sparing management for bulky cervical cancer using neoadjuvant transuterine arterialchemotherapy followed by vaginal trachelectomy[J].Int J Gynecol Cancer ,2012,22(6):1057?1062. [16]TSUJI N ,BUTSUHARA Y ,YOSHIKAWA H ,et al.Pregnancy after neoadjuvant chemotherapy followed by abdominal radical trachelectomy in stage ⅠB2cervical cancer :a case report[J].Gynecol Oncol Case Rep ,2012,4:13?15. [17]SATO S ,AOKI D ,KOBAYASHI H ,et al.Questionnaire survey of the current status of radical trachelectomy in Japan[J].Int J Clin Oncol , 2011,16(2):141?144. [18]ROBOVA H ,PLUTA M ,HREHORCAK M ,et al.High ?dose density chemotherapy followed by simple trachelectomy :full?term pregnancy[J].Int J Gynecol Cancer ,2009,18(6):1367?1371. [19]LANOWSKA M ,MANGLER M ,SPEISER D ,et al.Radical vaginal trachelectomy after laparoscopic staging and neoadjuvant chemotherapy in women with early?stage cervical cancer over 2cm :oncologic ,fertility ,and neonatal outcome in a series of 20patients[J].Int J Gynecol Cancer ,2014,24(3):586?593. [20]姚婷婷,陈勍,林仲秋.早期宫颈癌行经腹根治性宫颈切除后成功妊 娠2例报道[J].现代妇产科进展,2011,20(10):822?823. [21]DARGENT D ,FRANZOSI F ,ANSQUER Y ,et al.Extended trachelecto? my relapse :plea for patient involvement in the medical decision[J].Bull Cancer ,2002,89(12):1027?1030.[22]SCHLAERTH JB ,SPIRTOS NM.Radical trachelectomy and pelvic lymphadenectomy with uterine preservation in the treatment of cervical cancer[J].Am J Obstet Gynecol ,2003,188(1):29?34. (收稿日期:2017?11?04) 钠?葡萄糖共转运蛋白2抑制剂对糖尿病肾病保护作用的 研究进展 雷明静综述,钟 玲△审校(重庆医科大学附属第二医院肾内科,重庆400010) 【关键词】糖尿病肾病;钠;葡萄糖;载体蛋白质类;肾;血流动力学;综述 DOI :10.3969/j.issn.1009?5519.2018.12.022文献标识码:A 文章编号:1009?5519(2018)12?1839?03 钠?葡萄糖共转运蛋白2(SGLT2)抑制剂为一种新型降糖药,有降糖、降压、降尿蛋白、减轻体重、降尿酸、改善肾小球高滤过等作用。目前有研究提示,SGLT2抑制剂对糖尿病肾病(DN )患者降糖与降尿白蛋白作用不平行,提示其可能通过非糖依赖途径发挥肾脏保护作用。本文对SGLT2抑制剂对DN 保护作用、肾血流动力学、尿钠排泄、降尿白蛋白肌酐比等机制做一综述。1SGLT2抑制剂与DN 的关系 DN 为糖尿病患者的微血管重要并发症之一,其发病机制复杂,涉及的因素繁多,主要危险因素有糖尿病病程长、血糖控制不佳、肥胖、系统性高血压、脂质代谢紊乱等,单独的血糖升高不能完全解释其发生、发展,尽管改善生活方式和药物的使用[(降糖、降脂、降压,尤其是肾素?血管紧张素?醛固酮系统阻断剂(RAASi )]可以有效地控制这些危险因素,但DN 的发病率仍然居高不下,而且一旦出现肾功能异常,其进展速度要远快于非糖尿病性慢性肾脏病。在过去的20年里,一些新型的治疗策略,如双重或三重RASSi 用来减缓DN 患者肾功能进展,但是这些方案的效果有限,且其安全性受到质疑,迄今仍不推荐双重或三重RASSi 治疗DN [1]。因此,对于能够控制多种危险因素和可以保护肾脏结局的新疗法成为研究热点。 一种新型非胰岛素依赖途径的降糖药——SGLT2 抑制剂,其阻断近端小管中钠离子、葡萄糖重吸收,增加肾脏尿糖排泄并降低血糖[2]。研究发现,SGLT2抑制剂除降糖作用外,还有降低糖尿病患者血压、减轻体重、降低尿酸水平、改善肾小球高滤过、减少蛋白尿、增加尿钠离子排泄等作用。目前,美国食品和药品监督管理局(FDA )和欧洲药物管理局(EMA )批准了3种口服SGLT2抑制剂(坎格列净、达格列净、恩格列净),作为肾小球滤过率(eGFR )>30mL/(min·1.73m 2)的2型糖尿病患者可选择的二线或三线降糖治疗药物。2SGLT2抑制剂的肾脏保护作用独立于降糖效应 近年来,已有多项研究表明,SGLT2抑制剂肾脏保护作用可能通过非糖依赖途径,独立于其降糖作用。HEERSPINK 等[3]对1450例2型糖尿病患者分别使用坎格列净100、300mg 并与格列美脲6~8mg 进行对照,1年后,患者糖化血红蛋白(HbA1c )分别下降0.81%、0.82%、0.93%,2年后HbA1c 分别下降0.55%、0.65%、0.74%,而估计eGFR 分别降低3.3、0.5、0.9mL/(min×1.73m 2·年)(P <0.01)。对于尿白蛋白/肌酐(UACR )≥30mg/g 的患者,坎格列净300、100mg 对UACR 下降作用均优于格列美脲,提示坎格列净能延缓2型糖尿病患者肾功能下降,其肾脏保护作用独立于降糖作用。 PETRYKIV 等[4]对超过4000例2型糖尿病患者参与的为期24周的11个3期临床试验进行总结,发现 △ 通信作者,E?mail :536576113@https://www.360docs.net/doc/6512369707.html, 现代医药卫生2018年6月第34卷第12期J Mod Med Health ,June 2018,Vol.34,No.12· ·1839
葡萄糖转运载体测定方法
一.刷状缘膜囊的制备:(方法参照Kessler(1978),Shirazi-Beechey(1991)和Bauer (2001b)修正) 所有操作过程需在冰上或者4℃下进行。将冷冻样品进行称重,并在冷烧杯中用缓冲剂(100mM 甘露醇,2mM HEPES/Tris 缓冲剂,pH7.1)解冻。解冻后,在冷的有盖培养皿中用剪刀和镊子将组织样剪为1cm小块,将小块组织样重放入冷烧杯,然后将组织块悬液振动2次(设定为No.80),每次一分钟。用布氏漏斗过滤振动后溶液,溶液转入250mL量筒,记录滤液体积后转移至400mL烧杯中。将烧杯放置冰上,搅拌溶液。制作多个平行样以备进一步分析,这些处理后样品为匀浆液。已知浓度的氯化镁溶液加入到匀浆液中以达到10mM的终浓度,温和搅拌溶液20分钟,然后进行两种离心:5min,3000*g;30min,30000*g。利用20-G型检测针对剩余的颗粒进行再悬浮,缓冲液为100mM甘露醇+20mM HEPES/Tris,pH7.5。悬浮颗粒在组织研磨机(Potter-Elvehjam; Teflon/glass)中均匀搅拌十次后在30000*g离心30min。最终颗粒用23-G型检测针在500μL缓冲液中(300mM甘露醇,0.1mM硫酸镁,20mM HEPES/Tris,pH7.5)再次悬浮。悬浮颗粒(囊泡)由反复流过27-G型检测针10次的溶液制备均匀,避免出现气泡。将试样等分入冷冻管后-80℃保存。 二.检测 利用BSA(牛血清白蛋白)作为匀浆蛋白浓度和囊泡浓度的标准。麦芽糖酶是刷状缘膜富集度的标记酶,Truner 和Moran(1982)的方法测定麦芽糖酶活,使用25mM磷酸盐缓冲液(pH6.3)和30mM麦芽糖。释放的葡萄糖通过COBAS FaraⅡ(全自动生化仪)(Roche,Montclair,NJ)测定麦芽糖酶活表达为μmol product/min*mg of protein。囊泡中麦芽糖酶活的富集(enrichment)表示为囊泡麦芽糖酶活/匀浆麦芽糖酶活。 37℃下将5μL(50μg of protein)的小囊泡置于预热的微量离心管中,预热的培养基中包含100mM硫氰酸钠或硫氰酸钾、99.8mM甘露醇、20mM HEPES/Tris (pH7.5),再加入200μM葡萄糖(1.0μCi【U-14C】-D-glucose)。加入1mL冰冻终止溶液(150mL氯化钠,250μM根皮苷)3s后葡萄糖吸收被终止。移取0.9mL上述溶液,迅速通过0.45μm圆形纤维素薄膜。整个薄膜用终止溶液(5*1mL)清洗。将薄膜置于20mL 闪烁计数瓶中,瓶中加入12mL scintillation cocktail(Scintisafe Plus 50%LSC Cocktail)。样品通过Quantasmart软件用于放射性测定(闪仪Tri-Carb 2900TR/SL)。在Na+和K+存在的前提下,葡萄糖吸收的测定重复五次。钠依赖性葡萄糖的吸收通过硫氰酸钠孵化值减去硫氰酸钾孵化值得到。 三.免疫印迹
