Hydration of Dicyclopentadiene in the Presence of

Hydration of Dicyclopentadiene in the Presence of Cation Exchange Resin
Sandip Talwalkar,?Pramod Kumbhar,?and Sanjay Mahajani*,?
Department of Chemical Engineering,Indian Institute of Technology Bombay,Powai,400076Mumbai,India,and R &D Di V ision,Schenectady Herdillia Ltd.,Mumbai,India
In the present work the hydration of dicyclopentadiene (DCPD)has been studied in the presence of cation exchange resin catalyst.Ion exchange resin Amberlyst-15was found to offer the best performance with more than 95%selectivity toward cydecanol and conversion as high as 15%.The literature suggests that the zeolites are more active than ion exchange resin catalysts for such reactions.However,this is one of those few liquid phase hydrations for which ion exchange resins offer much better performance in terms of both rate and selectivity toward alcohol.An important finding of our earlier study on this reaction is that the properties of the ion exchange resin catalyst are modified during the course of reaction,which helps in improving the reaction kinetics.In the present work,the kinetic studies have been discussed in detail and issues such as catalyst reusability and reasons for the rate enhancement have been addressed.
1.Introduction
Cydecanol,the hydrated product of dicyclopentadiene,is an unsaturated bicyclic alcohol with applications in the manufacture of hydrocarbon resins and polyesters.It is also a potentially important chemical in the flavor and fragrance industry.It is an interesting monomer,in that the functionality of the double bond in combination with a hydroxyl group has many synthesis opportunities.1Generally it is prepared by liquid or vapor phase hydration of DCPD in the presence of acid catalyst as shown in Figure 1.2The syntheses of cydecanol with conventional acidic catalysts such as sulfuric acid,3niobic acid,4ion exchange resin,and metal oxides 4have been reported in the literature.Sulfuric acid,being a homogeneous acid,poses problems due to corrosion,disposal,and handling.Nafion is active but expensive.Niobic acid and metal oxides need special pretreat-ment,and the performance of these catalysts is sensitive to the pretreatment conditions.4However,the information available is not exhaustive and is insufficient for the design of a commercial reactor.There is a lack of exhaustive information in the open literature regarding this important reaction.
The main limitation of liquid phase reaction is the extremely poor miscibility of the two reactants,water and DCPD.The solubility of DCPD in water at 298K is less than 0.024v/v %.5Due to this,the reaction rates are substantially low and extremely active catalysts are required to obtain a significant conversion level.One alterative to enhance the rate of reaction is to introduce a cosolvent.6,7However,this approach of addition of an external component introduces extra process steps and increases the process cost.The reactions in biphasic (liquid -liquid mode)conditions are relatively clean but need active catalysts to compensate for the rate depletion caused by the lower solubility of olefins in water.The use of zeolite catalysts such as ZSM-5and -zeolites has been successfully investigated in the past for such reactions.8These catalysts are reported to have offered significant rates compared to the relatively mild ion exchange resins.
In our earlier work on this reaction with cation exchange resin,Amberlyst-15catalyst showed some interesting features.9It was
demonstrated that the catalyst can be effectively used in solid (catalyst)-liquid -liquid mode,and it was observed that the catalyst surface undergoes modification during the course of the reaction and in turn enhances the reaction rate without compromising on selectivity.The present work aims to inves-tigate the kinetic behavior of the system and develop a model,which may be used to design a commercial reactor.2.Experimental Section
2.1.Materials.Dicyclopentadiene (90%pure)was obtained from Lancaster Ltd.U.K.Rohm and Haas,France,supplied Amberlyst-15,and it was used without any prior treatment.2.2.Apparatus and Procedure.The batch reactions were conducted in a liquid -liquid -solid mode.A glass stirred reactor of 160mL capacity,equipped with an online temperature and agitation speed measuring facility,was used for this purpose.The reactor was inserted in an oil bath used to maintain the required reaction temperature.The desired quantities of catalyst and reactants were charged to the reactor,and the reaction mixture was heated to the desired temperature with slow stirring.As the reaction temperature was reached,the speed of agitation was increased up to the desired level and the corresponding time was considered the zero reaction time.The samples of both phases,organic and aqueous,were withdrawn at different time intervals to study the concentration change of reactants and products with respect to time.The reproducibility of the few representative experiments was checked by repeating the experiment three times,and the deviation from mean with error bars is given in section
3.6,Effect of Temperature.
2.3.Catalyst Characterization.The fresh and reused catalysts were characterized for surface area,ion exchange capacity,and wettability.The surface area of the catalyst was determined by nitrogen adsorption technique using the micro-flow BET technique (Smart Instruments Co.Ltd.,India).The concentration of active sites is determined by the procedure given in the literature.10A 1%NaCl solution was passed through the bed of the catalyst,and hydrochloric acid eluted was determined by titration with standard sodium hydroxide solution.
*To whom correspondence should be addressed.Tel.:+91-22-25767246.Fax:+91-22-25726895.E-mail:sanjaym@che.iitb.ac.in.?IIT Bombay.
?Schenectady Herdillia Ltd.
Figure 1.Reaction scheme for hydration of DCPD.
8024Ind.Eng.Chem.Res.2006,45,8024-8028
10.1021/ie060470n CCC:$33.50?2006American Chemical Society
Published on Web 11/01/2006
The contact angles were determined by using the Washburn method (GBX,Model DS,France),which uses a tensiometric method for measuring contact angles.The instrument is operated in two steps:in the first step the cell constant is determined by using a wetting liquid (hexane or heptane);i.e.,contact angle )0°.In the second step the contact angle of the solid with the test liquid (in this case water)is measured by measuring the amount of liquid that rises in the cell in which the solid is placed.Then the contact angle is calculated by applying the Washburn equation:11
where m is the mass of liquid that rises in the capillary cell in
time,t .F L ,ηL ,and γL are the liquid density,viscosity,and surface tension,respectively.C is the cell constant,and θis the contact angle to be determined.
2.4.Analysis.The reactants and products were analyzed using a gas chromatograph (GC-MAK-911)equipped with a flame ionization detector (FID).A 30m long capillary column,BP-1(SGE,Australia),was used to separate the different components in the reaction mixture using toluene as an external standard.The GC oven was operated under isothermal condition at 453K.The various components in the reaction mixture were characterized either by authentic sample and/or by gas chro-matography -mass spectrometry (GC -MS).The higher prod-ucts,which were not eluted in the GC,were calculated by difference in moles of DCPD reacted and moles of alcohol formed.The loss of DCPD to products other than alcohol was less than 5%in all experiments.Each sample was injected three times,and the maximum standard deviation,in the GC analysis,was less than
3.8%.3.Results and Discussion
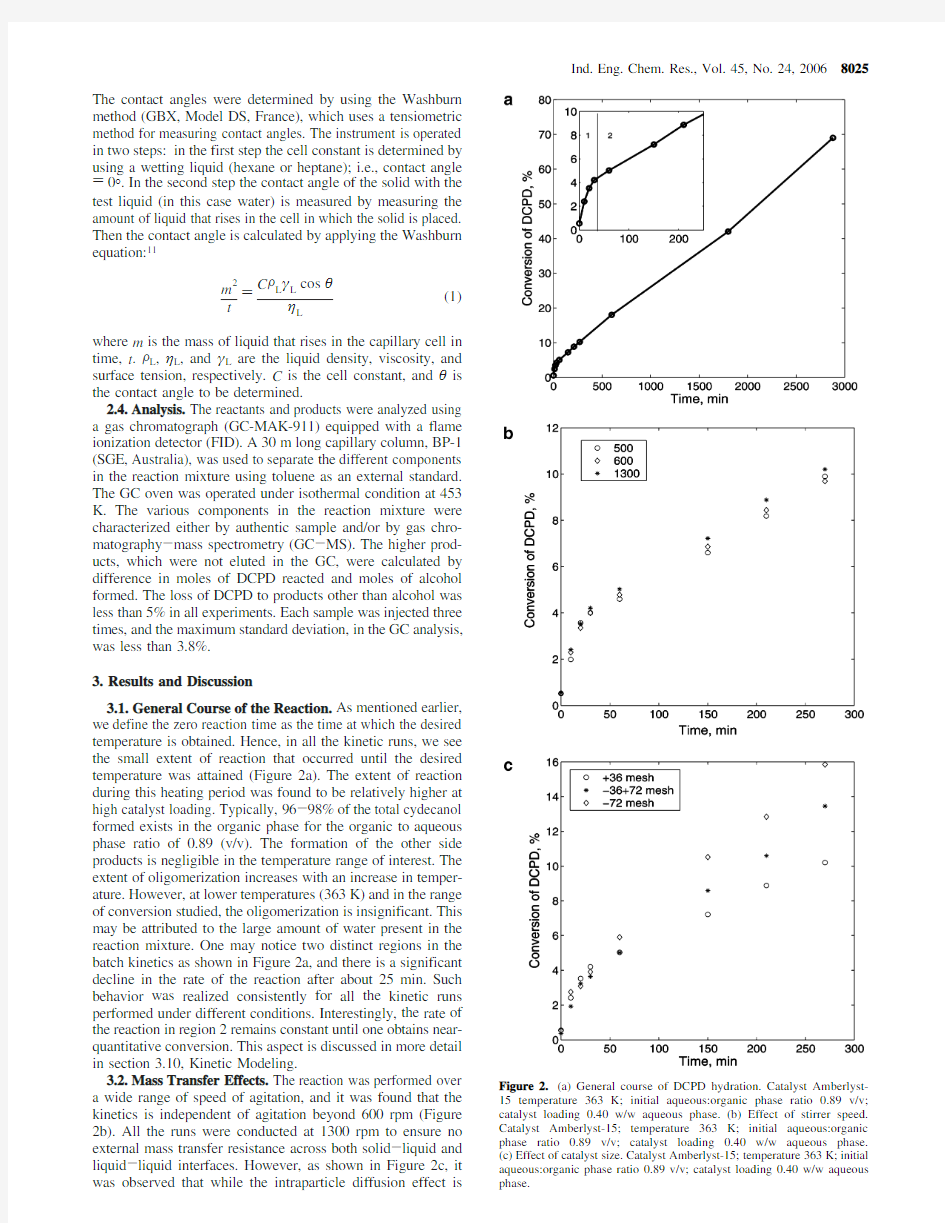
3.1.General Course of the Reaction.As mentioned earlier,we define the zero reaction time as the time at which the desired temperature is obtained.Hence,in all the kinetic runs,we see the small extent of reaction that occurred until the desired temperature was attained (Figure 2a).The extent of reaction during this heating period was found to be relatively higher at high catalyst loading.Typically,96-98%of the total cydecanol formed exists in the organic phase for the organic to aqueous phase ratio of 0.89(v/v).The formation of the other side products is negligible in the temperature range of interest.The extent of oligomerization increases with an increase in temper-ature.However,at lower temperatures (363K)and in the range of conversion studied,the oligomerization is insignificant.This may be attributed to the large amount of water present in the reaction mixture.One may notice two distinct regions in the batch kinetics as shown in Figure 2a,and there is a significant decline in the rate of the reaction after about 25min.Such behavior was realized consistently for all the kinetic runs performed under different conditions.Interestingly,the rate of the reaction in region 2remains constant until one obtains near-quantitative conversion.This aspect is discussed in more detail in section 3.10,Kinetic Modeling.
3.2.Mass Transfer Effects.The reaction was performed over a wide range of speed of agitation,and it was found that the kinetics is independent of agitation beyond 600rpm (Figure 2b).All the runs were conducted at 1300rpm to ensure no external mass transfer resistance across both solid -liquid and liquid -liquid interfaces.However,as shown in Figure 2c,it was observed that while the intraparticle diffusion effect is
m 2t )C F L γL cos θηL
(1)
Figure 2.(a)General course of DCPD hydration.Catalyst Amberlyst-15temperature 363K;initial aqueous:organic phase ratio 0.89v/v;catalyst loading 0.40w/w aqueous phase.(b)Effect of stirrer speed.Catalyst Amberlyst-15;temperature 363K;initial aqueous:organic phase ratio 0.89v/v;catalyst loading 0.40w/w aqueous phase.(c)Effect of catalyst size.Catalyst Amberlyst-15;temperature 363K;initial aqueous:organic phase ratio 0.89v/v;catalyst loading 0.40w/w aqueous phase.
Ind.Eng.Chem.Res.,Vol.45,No.24,20068025
negligible in region 1,the same is substantial in region 2.It was observed during the subsequent kinetic analysis that the rate of reaction in region 2is insensitive to almost all the operating parameters,and activation energy for this region is almost zero.Thus,there is considerable intraparticle diffusion resistance in region 2.However,using catalysts at the smaller size is less preferred due to difficulties in the industrial operations;therefore,the kinetic analysis is performed with the commercially available size.
3.3.Mode of the Reaction.Since DCPD is immiscible in water,the reaction takes place in triphasic solid -liquid -liquid mode.An interesting observation was made while studying the kinetic runs.If the catalyst is soaked in water followed by addition of DCPD (mode 2),the reaction in the initial period is much slower than when the reactor is first charged with catalyst and DCPD followed by water (mode 1)(see Figure 3).Because of the presence of water,DCPD oligomerization is much slower.However,when the catalyst is first exposed to DCPD,it is conjectured that the instantaneous oligomerization takes place and the presence of oligomers on the catalyst surface makes it hydrophobic.This hydrophobicity,in our opinion,is responsible for the enhanced rate and conversion.This hypothesis is supported by the fact that when the catalyst from mode 2is reused with the fresh reactants,the initial reaction rate observed is higher than when the fresh catalyst is used under similar conditions.This effect is discussed in detail in section 3.7,Catalyst Reusability.All the runs in the present work,unless otherwise mentioned,have been performed in mode 1.
3.4.Effect of Catalyst Loading.The reactions were per-formed over a wide range of catalyst loading (0.10-0.40w/w aqueous phase),and as expected,it was observed that the rate of reaction increases with the catalyst loading.Figure 4shows the plot of conversion vs time at different catalyst loadings.The initial rate of reaction increases linearly with an increase in catalyst loading.Again,as mentioned before,two distinct regions in the batch kinetics are clearly evident.As expected,the rates in both regions appear to be a function of catalyst loading.
3.5.Effect of Aqueous Phase Holdup.The volume ratio of aqueous phase to organic phase was varied over the range 0.43-2.33under otherwise similar values of catalyst loadings,total reaction volume,and reaction temperature.Figure 5shows the
influence of aqueous phase holdup on DCPD conversion.It was observed that the rate of reaction increases with an increase in aqueous to organic phase ratio.This effect is difficult to explain at this stage,and only a detailed investigation on the catalysis will throw light on this observation.However,it should be noted that the rate in region 2is almost independent of the phase ratio.3.6.Effect of Temperature.The extractive (liquid -liquid)reactions are highly influenced by temperature as both intrinsic rate constants and distribution coefficients are strong functions of temperature.DCPD hydration was studied over the temper-ature range of 346-368K.Figure 6shows the effect of temperature and standard deviation in the experimental data on the conversion vs time plot under otherwise similar conditions.The rate increases significantly with an increase in temperature.The rate in region 2is less sensitive to the change in temperature.At high temperature (373K),it was observed that the reaction mixture acquires a dark brownish color due to the formation of oligomers of DCPD.Hence,all the reactions were conducted at 363K.It was also observed that,with an increase in the reaction temperature,the color of the used catalyst changes
from
Figure 3.Effect of mode of addition of reactants on DCPD hydration.Catalyst Amberlyst-15;temperature 363K;initial aqueous:organic phase ratio 0.89v/v;catalyst loading 0.40w/w aqueous
phase.
Figure 4.Effect of catalyst loading on DCPD hydration.Catalyst Amberlyst-15;temperature 363K;initial aqueous:organic phase ratio 0.89v/v.Catalyst loading is with respect to the aqueous phase,in w/w aqueous
phase.
Figure 5.Effect of initial aqueous to organic phase ratio on DCPD hydration.Catalyst Amberlyst-15;temperature 363K;catalyst loading 0.40w/w aqueous phase.Initial aqueous:organic phase ratio in v/v.
8026Ind.Eng.Chem.Res.,Vol.45,No.24,2006
light brown to dark blackish brown possibly due to the formation of higher oligomers.
3.7.Catalyst Reusability.It is a well-known fact that the reactions associated with olefins encounter the problems of catalyst deactivation due to formation of dimers,oligomers,and carbonaceous material.12The kinetics for the used catalyst is compared with that for the fresh catalyst in Figure 7.The rate of reaction was found to increase after every reuse for a few intial runs of typical batch time of
4.5h,and then it remained constant.9As discussed earlier,the color of the reused catalyst was found to be dark brown.In our earlier work,9we have observed that,with reuse,the hydrophobicity of the catalyst increased,and if the used catalyst is placed in the two-phase mixture,it slowly migrates from the aqueous phase toward the organic phase.ESEM pictures of fresh and used catalysts clearly show that the there is a uniform coating on the used catalyst as discussed in our earlier work.9
https://www.360docs.net/doc/d317537079.html,parison of Different Catalysts.Our previous experience 8,13,14and the literature 15suggest that zeolites perform better than ion exchange resin catalyst for many hydrations.
However,we have observed that Amberlyst-15and Indion-130are the best among all catalysts,with selectivity toward cydecanol more than 95%at the conversion on the order of 4%.The side products obtained with ZSM-5and zeolite- were significant under the conditions studied with conversion of DCPD on the order of 7%and 10%and selectivity toward cydecanol being 4%and 5%,respectively.Thus,in our opinion this is one of those few liquid phase olefin hydrations for which ion exchange resins offer much better performance in terms of both rate and selectivity toward the alcohol.
3.9.Catalyst Characterization.Our assumption of increase in hydrophobicity of the catalyst is supported by the contact angle measurement of catalyst with usage.The results show that the contact angle and hence hydrophobicity of the catalyst increases with usage.The results are summarized in Table 1.As expected,the surface area and the available concentration of the active sites reduce after every reuse.On the other hand,it is remarkable that there is no adverse effect of this change on the reaction kinetics.
3.10.Kinetic Modeling.It is difficult to develop a rigorous kinetic model for this reacting system as the catalyst is modified during the course of the reaction and exact mechanism of the same is not yet well understood.However,based on the results obtained in these studies,a working kinetic rate equation,useful for reactor design,has been proposed here.As mentioned before,the course of reaction may be divided into two different regions.In the initial period (region 1)the apparent kinetics can be given by
where r is the rate of reaction in s -1and M cat is mass of catalyst in kg.The estimated values of k °′and E °are 6.035×107kg -1s -1and 74.12kJ mol -1,respectively.
In about 25min of the batch time the reaction changes its course.It is conjectured that the catalyst undergoes changes in this period.The oligomerization reaction,which is responsible for the coating of the catalyst surface,takes place predominantly in the first 25min.The kinetics thereafter can be simply given by a zero-order reaction with an apparent rate constant of 2.735×10-2min -1mol ?L -1for a commercially available Amberlyst-15with a particle size of 600-800μm.The activation energy for this region of kinetics is close to zero as the rate was found to be a weak function of temperature.It can be seen from Figure 2c that the rate in region 2,which is otherwise insensitive to the changes in other parameters,is a strong function of particle size.This leads us to the conclusion that in region 1the reaction is controlled by intrinsic kinetics whereas,due to surface and pore structure modification in the catalyst,intraparticle diffusion plays an important role in region 2.An independent analysis on pore size distribution also shows a decline in the average pore size,which supports the above inference.9
The comparison between modeled and measured fractional conversion of DCPD for different temperatures and catalyst loadings is shown in Figures 8and 9.The model predictions are in good agreement with the experimental values in region 1.However,the model slightly underpredicts the rate in region 2.
4.Conclusions
Hydration of DCPD has been studied in the presence of a cation exchange resin.The batch reaction kinetics of this reaction may be divided into two regions:the initial region in
which
Figure 6.Effect of temperature on DCPD hydration.Catalyst Amberlyst-15;Catalyst loading 0.40w/w aqueous phase;initial aqueous:organic phase ratio 0.89v/v.Temperatures in
K.
Figure 7.Catalyst reusability for DCPD.Catalyst Amberlyst-15;temper-ature 363K;initial aqueous:organic phase ratio 2.38v/v;catalyst loading 0.14w/w aqueous phase;typical batch time 4.5h.9
r )M cat k °′exp
(-E °RT )
(2)
Ind.Eng.Chem.Res.,Vol.45,No.24,20068027
kinetics is sensitive to the change in parameters such as temperature,phase ratio,and catalyst loading and the second region in which rate becomes insensitive to almost all the parameters except catalyst loading and the particle size.The catalyst is modified during the course of reaction and offers improved kinetics.The reaction is zero order at longer reaction time and also insensitive to the change in temperature.Further investigations on catalysis are necessary to further capture this effect in a kinetic model.Manipulation of the catalyst hydro-phobicity/hydrophilicity through variation in Si/Al to improve catalysis is common for inorganic catalysts like zeolites;however,ion exchange resins have not been studied well in this direction and this work provides important input in this regard.A working kinetic model is given which gives good agreement with observed values in the initial period.It is difficult to develop a rigorous kinetic model at this stage for
this system due to the complex phenomenon of surface modification.Acknowledgment
We thank Richard Wall of CXI (Texmark),USA,for supplying a sample of cydecanol.Literature Cited
(1)Texmark,a Division of Chemical Exchange Industries,Inc.(CXI)Home https://www.360docs.net/doc/d317537079.html,/press-cydecanol.html (accessed Dec 2005).(2)Okazaki,S.;Kasano,K.;Kenji,U.Catalyst for dicyclopentadine hydration reaction.Japanese Patent 1,099,648,1989.
(3)Sasaki,A.;Saito,T.;Kikuchi,N.Production of tricyclo (5,2,1,02.6)-3-decen-8(or 9)-ol.Japanese Patent 6,320,8542,1988.
(4)Okazaki,S.;Harada,H.Vapor-phase hydration of dicyclopentadine catalyzed by niobic acid.Chem.Lett.1988,8,1313.
(5)UNEP Chemicals’Programme Home Page,www.chem.unep.ch/irptc/slids/OECDSIDS/66636.pdf (accessed June 2005).
(6)Chakrabarti, A.;Sharma,M.M.Ion-exchange resin catalyzed hydration of R -methylstyrene and etherification of R -methylstyrene with methanol.React.Polym.1992,18,117.
(7)Panneman,H.J.;Beenackers,A.A.C.M.Effect on the hydration of cyclohexene catalyzed by strong ion exchange resins I:Solubility of cyclohexene in aqueous sulfolane mixture.Ind.Eng.Chem.Res.1992,31,1226;Effect on the hydration of cyclohexene catalyzed by strong ion exchange resins II:Effect of sulfolane on reaction kinetics.Ind.Eng.Chem.Res.1992,31,1426;Effect on the hydration of cyclohexene catalyzed by strong ion exchange resins III:Effect of sulfolane on equilibrium conversion.Ind.Eng.Chem.Res.1992,31,1433.
(8)Zhang,H.;Mahajani,S.M.;Sharma,M.;Sridhar,T.Hydration of cyclohexene with solid acid catalysts.Chem.Eng.Sci.2002,66,316.(9)Talwalkar,S.;Kumbhar,P.;Mahajani,S.In situ coating on cation exchange resin catalyst,Amberlyst-15,and its impact on the hydration of https://www.360docs.net/doc/d317537079.html,mun.2006,7,717.
(10)Malshe,V.C.;Sujatha,E.S.Regeneration and reuse of cation-exchange resin catalyst used in alkylation of phenol.React.Funct.Polym.1997,35,159.
(11)User manual for powder wettability,GBX Instrument.
(12)Ishada,H.Liquid phase hydration of cyclohexene with zeolites.Catal.Sur V .Jpn.1997,1,241.
(13)Mahajani,S.M.;Sharma,M.M.;Sridhar,T.Extractive hydration of n -butene with solid acid catalysts in the liquid phase and under supercritical conditions.Chem.Eng.Sci.2001,56,5625.
(14)Mahajani,S.M.;Sharma,M.M.;Sridhar,T.Direct hydration of propylene in liquid phase and under supercritical conditions in the presence of solid acid catalysts.Chem.Eng.Sci.2002,57,4877.
(15)Venuto,https://www.360docs.net/doc/d317537079.html,anic catalysis over zeolites:A perspective on reaction paths within micropores.Microporous Mater.1994,2,296.
Recei V ed for re V iew April 14,2006
Re V ised manuscript recei V ed August 14,2006
Accepted September 16,2006
IE060470N
Table 1.Properties of Fresh and Used Catalyst 9
property
fresh after 2runs after 4runs after 10runs surface area,m 2/g 34.8529.9818.4615.43contact angle,deg
068.289.990.0concentration of active sites,mequiv/g
4.2
3.60
2.82
2.35
Figure https://www.360docs.net/doc/d317537079.html,parison between measured and modeled values for different temperatures.(])346K;(O )355K;(4)363K;other conditions same as Figure
2a.
Figure https://www.360docs.net/doc/d317537079.html,parison between measured and modeled values for different catalyst loadings.(])0.10w/w aqueous phase;(O )0.25w/w aqueous phase;(4)0.40w/w aqueous phase;other conditions same as Figure 2a.
8028Ind.Eng.Chem.Res.,Vol.45,No.24,2006
英语中的比较级与最高级 详解
比较级与最高级 1.as...as 与(not) as(so)...as as...as...句型中,as的词性 第一个as是副词,用在形容词和副词的原级前,常译为“同样地”。第二个as是连词,连接与前面句子结构相同的一个句子(相同部分常省略),可译为“同..... He is as tall as his brother is (tall) . (后面的as 为连词) 只有在否定句中,第一个as才可换为so 改错: He is so tall as his brother.(X) 2.在比较状语从句中,主句和从句的句式结构一般是相同的 与as...as 句式中第二个as一样,than 也是连词。as和than这两个连词后面的从句的结构与前面的句子大部分情况下结构是相同的,相同部分可以省略。 He picked more apples than she did. 完整的表达为: He picked more apples than she picked apples. 后而的picked apples和前面相同,用did 替代。 He walked as slowly as she did.完整表达为: He walked as slowly as she walked slowly. she后面walked slowly与前面相同,用did替代。
3.谓语的替代 在as和than 引导的比较状语从句中,由于句式同前面 主句相同,为避免重复,常把主句中出现而从句中又出现的动词用do的适当形式来代替。 John speaks German as fluently as Mary does. 4.前后的比较对象应一致 不管后面连词是than 还是as,前后的比较对象应一致。The weather of Beijing is colder than Guangzhou. x than前面比较对象是“天气”,than 后面比较对象是“广州”,不能相比较。应改为: The weather of Bejing is colder than that of Guangzhou. 再如: His handwriting is as good as me. 应改为: His handwriting is as good as mine. 5.可以修饰比较级的词 常用来修饰比较级的词或短语有: Much,even,far,a little,a lot,a bit,by far,rather,any,still,a great deal等。 by far的用法: 用于强调,意为“...得多”“最最...”“显然”等,可修饰形容词或副词的比较级和最高级,通常置于其后,但是若比较级或最高级前有冠词,则可置于其前或其后。
The way常见用法
The way 的用法 Ⅰ常见用法: 1)the way+ that 2)the way + in which(最为正式的用法) 3)the way + 省略(最为自然的用法) 举例:I like the way in which he talks. I like the way that he talks. I like the way he talks. Ⅱ习惯用法: 在当代美国英语中,the way用作为副词的对格,“the way+ 从句”实际上相当于一个状语从句来修饰整个句子。 1)The way =as I am talking to you just the way I’d talk to my own child. He did not do it the way his friends did. Most fruits are naturally sweet and we can eat them just the way they are—all we have to do is to clean and peel them. 2)The way= according to the way/ judging from the way The way you answer the question, you are an excellent student. The way most people look at you, you’d think trash man is a monster. 3)The way =how/ how much No one can imagine the way he missed her. 4)The way =because
人教版(新目标)初中英语形容词与副词的比较级与最高级
人教版(新目标)初中英语形容词与副词的比较级与最高级 (一)规则变化: 1.绝大多数的单音节和少数双音节词,加词尾-er ,-est tall—taller—tallest 2.以不发音的e结尾的单音节词和少数以-le结尾的双音节词只加-r,-st nice—nicer—nicest , able—abler—ablest 3.以一个辅音字母结尾的重读闭音节词或少数双音节词,双写结尾的辅音字母,再加-er,-est big—bigger—biggest 4.以辅音字母加y结尾的双音节词,改y为i再加-er,-est easy—easier—easiest 5.少数以-er,-ow结尾的双音节词末尾加-er,-est clever—cleverer—cleverest, narrow—narrower—narrowest 6.其他双音节词和多音节词,在前面加more,most来构成比较级和最高级 easily—more easily—most easily (二)不规则变化 常见的有: good / well—better—best ; bad (ly)/ ill—worse—worst ; old—older/elder—oldest/eldest many / much—more—most ; little—less—least ; far—farther/further—farthest/furthest
用法: 1.原级比较:as + adj./adv. +as(否定为not so/as + adj./adv. +as)当as… as中间有名字时,采用as + adj. + a + n.或as + many / much + n. This is as good an example as the other is . I can carry as much paper as you can. 表示倍数的词或其他程度副词做修饰语时放在as的前面 This room is twice as big as that one. 倍数+as+adj.+as = 倍数+the +n.+of Your room is twice as larger as mine. = Your room is twice the size of mine. 2.比较级+ than 比较级前可加程度状语much, still, even, far, a lot, a little, three years. five times,20%等 He is three years older than I (am). 表示“(两个中)较……的那个”时,比较级前常加the(后面有名字时前面才能加冠词) He is the taller of the two brothers. / He is taller than his two brothers. Which is larger, Canada or Australia? / Which is the larger country, Canada or Australia? 可用比较级形式表示最高级概念,关键是要用或或否定词等把一事物(或人)与其他同类事物(或人)相分离 He is taller than any other boy / anybody else.
英语中的比较级和最高级
大多数形容词有三种形式,原级,比较级和最高级, 以表示形容词说明的性质在程度上的不同。 形容词的原级: 形容词的原级形式就是词典中出现的形容词的原形。例如: poor tall great glad bad 形容词的比较级和最高级: 形容词的比较级和最高级形式是在形容词的原级形式的基础上变化的。分为规则变化和不规则变化。 规则变化如下: 1) 单音节形容词的比较级和最高级形式是在词尾加 -er 和 -est 构成。 great (原级) (比较级) (最高级) 2) 以 -e 结尾的单音节形容词的比较级和最高级是在词尾加 -r 和 -st 构成。wide (原级) (比较级) (最高级) 3)少数以-y, -er, -ow, -ble结尾的双音节形容词的比较级和最高级是在词尾加 -er 和 -est 构成。 clever(原级) (比较级) (最高级) 4) 以 -y 结尾,但 -y 前是辅音字母的形容词的比较级和最高级是把 -y 去掉,加上 -ier 和-est 构成. happy (原形) (比较级) (最高级) 5) 以一个辅音字母结尾其前面的元音字母发短元音的形容词的比较级和最高级是双写该辅音字母然后再加 -er和-est。 big (原级) (比较级) (最高级) 6) 双音节和多音节形容词的比较级和最高级需用more 和 most 加在形容词前面来构成。 beautiful (原级) (比较级) (比较级) difficult (原级) (最高级) (最高级) 常用的不规则变化的形容词的比较级和最高级: 原级------比较级------最高级 good------better------best many------more------most much------more------most bad------worse------worst far------farther, further------farthest, furthest 形容词前如加 less 和 least 则表示"较不"和"最不 形容词比较级的用法: 形容词的比较级用于两个人或事物的比较,其结构形式如下: 主语+谓语(系动词)+ 形容词比较级+than+ 对比成分。也就是, 含有形容词比较级的主句+than+从句。注意从句常常省去意义上和主句相同的部分, 而只剩下对比的成分。
The way的用法及其含义(二)
The way的用法及其含义(二) 二、the way在句中的语法作用 the way在句中可以作主语、宾语或表语: 1.作主语 The way you are doing it is completely crazy.你这个干法简直发疯。 The way she puts on that accent really irritates me. 她故意操那种口音的样子实在令我恼火。The way she behaved towards him was utterly ruthless. 她对待他真是无情至极。 Words are important, but the way a person stands, folds his or her arms or moves his or her hands can also give us information about his or her feelings. 言语固然重要,但人的站姿,抱臂的方式和手势也回告诉我们他(她)的情感。 2.作宾语 I hate the way she stared at me.我讨厌她盯我看的样子。 We like the way that her hair hangs down.我们喜欢她的头发笔直地垂下来。 You could tell she was foreign by the way she was dressed. 从她的穿著就可以看出她是外国人。 She could not hide her amusement at the way he was dancing. 她见他跳舞的姿势,忍俊不禁。 3.作表语 This is the way the accident happened.这就是事故如何发生的。 Believe it or not, that's the way it is. 信不信由你, 反正事情就是这样。 That's the way I look at it, too. 我也是这么想。 That was the way minority nationalities were treated in old China. 那就是少数民族在旧中
英语比较级和最高级的用法归纳
英语比较级和最高级的用法归纳 在学习英语过程中,会遇到很多的语法问题,比如比较级和最高级的用法,对于 这些语法你能够掌握吗?下面是小编整理的英语比较级和最高级的用法,欢迎阅读! 英语比较级和最高级的用法 一、形容词、副词的比较级和最高级的构成规则 1.一般单音节词和少数以-er,-ow结尾的双音节词,比较级在后面加-er,最高级 在后面加-est; (1)单音节词 如:small→smaller→smallest short→shorter→shortest tall→taller→tallest great→greater→greatest (2)双音节词 如:clever→cleverer→cleverest narrow→narrower→narrowest 2.以不发音e结尾的单音节词,比较在原级后加-r,最高级在原级后加-st; 如:large→larger→largest nice→nicer→nicest able→abler→ablest 3.在重读闭音节(即:辅音+元音+辅音)中,先双写末尾的辅音字母,比较级加-er,最高级加-est; 如:big→bigger→biggest hot→hotter→hottest fat→fatter→fattest 4.以“辅音字母+y”结尾的双音节词,把y改为i,比较级加-er,最高级加-est; 如:easy→easier→easiest heavy→heavier→heaviest busy→busier→busiest happy→happier→happiest 5.其他双音节词和多音节词,比较级在前面加more,最高级在前面加most; 如:bea utiful→more beautiful→most beautiful different→more different→most different easily→more easily→most easily 注意:(1)形容词最高级前通常必须用定冠词 the,副词最高级前可不用。 例句: The Sahara is the biggest desert in the world. (2) 形容词most前面没有the,不表示最高级的含义,只表示"非常"。 It is a most important problem. =It is a very important problem.
(完整版)the的用法
定冠词the的用法: 定冠词the与指示代词this ,that同源,有“那(这)个”的意思,但较弱,可以和一个名词连用,来表示某个或某些特定的人或东西. (1)特指双方都明白的人或物 Take the medicine.把药吃了. (2)上文提到过的人或事 He bought a house.他买了幢房子. I've been to the house.我去过那幢房子. (3)指世界上独一无二的事物 the sun ,the sky ,the moon, the earth (4)单数名词连用表示一类事物 the dollar 美元 the fox 狐狸 或与形容词或分词连用,表示一类人 the rich 富人 the living 生者 (5)用在序数词和形容词最高级,及形容词等前面 Where do you live?你住在哪? I live on the second floor.我住在二楼. That's the very thing I've been looking for.那正是我要找的东西. (6)与复数名词连用,指整个群体 They are the teachers of this school.(指全体教师) They are teachers of this school.(指部分教师) (7)表示所有,相当于物主代词,用在表示身体部位的名词前 She caught me by the arm.她抓住了我的手臂. (8)用在某些有普通名词构成的国家名称,机关团体,阶级等专有名词前 the People's Republic of China 中华人民共和国 the United States 美国 (9)用在表示乐器的名词前 She plays the piano.她会弹钢琴. (10)用在姓氏的复数名词之前,表示一家人 the Greens 格林一家人(或格林夫妇) (11)用在惯用语中 in the day, in the morning... the day before yesterday, the next morning... in the sky... in the dark... in the end... on the whole, by the way...
英语比较级和最高级的用法
More than的用法 A. “More than+名词”表示“不仅仅是” 1)Modern science is more than a large amount of information. 2)Jason is more than a lecturer; he is a writer, too. 3) We need more than material wealth to build our country.建设我们国家,不仅仅需要物质财富. B. “More than+数词”含“以上”或“不止”之意,如: 4)I have known David for more than 20 years. 5)Let's carry out the test with more than the sample copy. 6) More than one person has made this suggestion. 不止一人提过这个建议. C. “More than+形容词”等于“很”或“非常”的意思,如: 7)In doing scientific experiments, one must be more than careful with the instruments. 8)I assure you I am more than glad to help you. D. more than + (that)从句,其基本意义是“超过(=over)”,但可译成“简直不”“远非”.难以,完全不能(其后通常连用情态动词can) 9) That is more than I can understand . 那非我所能懂的. 10) That is more than I can tell. 那事我实在不明白。 11) The heat there was more than he could stand. 那儿的炎热程度是他所不能忍受的 此外,“more than”也在一些惯用语中出现,如: more...than 的用法 1. 比……多,比……更 He has more books than me. 他的书比我多。 He is more careful than the others. 他比其他人更仔细。 2. 与其……不如 He is more lucky than clever. 与其说他聪明,不如说他幸运。 He is more (a)scholar than (a)teacher. 与其说他是位教师,不如说他是位学者。 注:该句型主要用于同一个人或物在两个不同性质或特征等方面的比较,其中的比较级必须用加more 的形式,不能用加词尾-er 的形式。 No more than/not more than 1. no more than 的意思是“仅仅”“只有”“最多不超过”,强调少。如: --This test takes no more than thirty minutes. 这个测验只要30分钟。 --The pub was no more than half full. 该酒吧的上座率最多不超过五成。-For thirty years,he had done no more than he (had)needed to. 30年来,他只干了他需要干的工作。 2. not more than 为more than (多于)的否定式,其意为“不多于”“不超过”。如:Not more than 10 guests came to her birthday party. 来参加她的生日宴会的客人不超过十人。 比较: She has no more than three hats. 她只有3顶帽子。(太少了) She has not more than three hats. 她至多有3顶帽子。(也许不到3顶帽子) I have no more than five yuan in my pocket. 我口袋里的钱最多不过5元。(言其少) I have not more than five yuan in my pocket. 我口袋里的钱不多于5元。(也许不到5元) more than, less than 的用法 1. (指数量)不到,不足 It’s less than half an hour’s drive from here. 开车到那里不到半个钟头。 In less than an hour he finished the work. 没要上一个小时,他就完成了工作。 2. 比……(小)少 She eats less than she should. 她吃得比她应该吃的少。 Half the group felt they spent less than average. 半数人觉得他们的花费低于平均水平。 more…than,/no more than/not more than (1)Mr.Li is ________ a professor; he is also a famous scientist. (2)As I had ________ five dollars with me, I couldn’t afford the new jacket then. (3)He had to work at the age of ________ twelve. (4)There were ________ ten chairs in the room.However, the number of the children is twelve. (5)If you tel l your father what you’ve done, he’ll be ________ angry. (6)-What did you think of this novel? -I was disappointed to find it ________ interesting ________ that one. 倍数表达法 1. “倍数+形容词(或副词)的比较级+than+从句”表示“A比B大(长、高、宽等)多少倍” This rope is twice longer than that one.这根绳是那根绳的三倍(比那根绳长两倍)。The car runs twice faster than that truck.这辆小车的速度比那辆卡车快两倍(是那辆卡车的三倍)。 2. “倍数+as+形容词或副词的原级+as+从句”表示“A正好是B的多少倍”。
“the way+从句”结构的意义及用法
“theway+从句”结构的意义及用法 首先让我们来看下面这个句子: Read the followingpassageand talkabout it wi th your classmates.Try totell whatyou think of Tom and ofthe way the childrentreated him. 在这个句子中,the way是先行词,后面是省略了关系副词that或in which的定语从句。 下面我们将叙述“the way+从句”结构的用法。 1.the way之后,引导定语从句的关系词是that而不是how,因此,<<现代英语惯用法词典>>中所给出的下面两个句子是错误的:This is thewayhowithappened. This is the way how he always treats me. 2.在正式语体中,that可被in which所代替;在非正式语体中,that则往往省略。由此我们得到theway后接定语从句时的三种模式:1) the way+that-从句2)the way +in which-从句3) the way +从句 例如:The way(in which ,that) thesecomrade slookatproblems is wrong.这些同志看问题的方法
不对。 Theway(that ,in which)you’re doingit is comple tely crazy.你这么个干法,简直发疯。 Weadmired him for theway inwhich he facesdifficulties. Wallace and Darwingreed on the way inwhi ch different forms of life had begun.华莱士和达尔文对不同类型的生物是如何起源的持相同的观点。 This is the way(that) hedid it. I likedthe way(that) sheorganized the meeting. 3.theway(that)有时可以与how(作“如何”解)通用。例如: That’s the way(that) shespoke. = That’s how shespoke.
初中英语比较级和最高级讲解与练习
初中英语比较级和最高级讲解与练习 形容词比较级和最高级 一.绝大多数形容词有三种形式,原级,比较级和最高级, 以表示形容词说明的性质在程度上的不同。 1. 形容词的原级: 形容词的原级形式就是词典中出现的形容词的原形。例如: poor tall great glad bad 2. 形容词的比较级和最高级: 形容词的比较级和最高级形式是在形容词的原级形式的基 础上变化的。分为规则变化和不规则变化。 二.形容词比较级和最高级规则变化如下: 1) 单音节形容词的比较级和最高级形式是在词尾加-er 和-est 构成。 great (原级) greater(比较级) greatest(最高级) 2) 以-e 结尾的单音节形容词的比较级和最高级是在词尾加-r 和-st 构成。 wide (原级) wider (比较级) widest (最高级) 3) 少数以-y, -er, -ow, -ble结尾的双音节形容词的比较级和最高级是在词尾加 -er 和-est构成。 clever(原级) cleverer(比较级) cleverest(最高级), slow(原级) slower(比较级) slowest (最高级) 4) 以-y 结尾,但-y 前是辅音字母的形容词的比较级和最高级是把-y 去掉,加上-ier 和-est 构成. happy (原形) happier (比较级) happiest (最高级) 5) 以一个辅音字母结尾其前面的元音字母发短元音的形容词的比较级和最高级是双写该 辅音字母然后再加-er和-est。 原形比较级最高级原形比较级最高级 big bigger biggest hot hotter hottest red redder reddest thin thinner thinnest 6) 双音节和多音节形容词的比较级和最高级需用more 和most 加在形容词前面来构 成。 原形比较级最高级 careful careful more careful most careful difficult more difficult most difficult delicious more delicious most delicious 7)常用的不规则变化的形容词的比较级和最高级: 原级比较级最高级 good better best 好的 well better best 身体好的 bad worse worst 坏的 ill worse worst 病的 many more most 许多 much more most 许多 few less least 少数几个 little less least 少数一点儿 (little littler littlest 小的) far further furthest 远(指更进一步,深度。亦可指更远) far farther farthest 远(指更远,路程)
way 用法
表示“方式”、“方法”,注意以下用法: 1.表示用某种方法或按某种方式,通常用介词in(此介词有时可省略)。如: Do it (in) your own way. 按你自己的方法做吧。 Please do not talk (in) that way. 请不要那样说。 2.表示做某事的方式或方法,其后可接不定式或of doing sth。 如: It’s the best way of studying [to study] English. 这是学习英语的最好方法。 There are different ways to do [of doing] it. 做这事有不同的办法。 3.其后通常可直接跟一个定语从句(不用任何引导词),也可跟由that 或in which 引导的定语从句,但是其后的从句不能由how 来引导。如: 我不喜欢他说话的态度。 正:I don’t like the way he spoke. 正:I don’t like the way that he spoke. 正:I don’t like the way in which he spoke. 误:I don’t like the way how he spoke. 4.注意以下各句the way 的用法: That’s the way (=how) he spoke. 那就是他说话的方式。 Nobody else loves you the way(=as) I do. 没有人像我这样爱你。 The way (=According as) you are studying now, you won’tmake much progress. 根据你现在学习情况来看,你不会有多大的进步。 2007年陕西省高考英语中有这样一道单项填空题: ——I think he is taking an active part insocial work. ——I agree with you_____. A、in a way B、on the way C、by the way D、in the way 此题答案选A。要想弄清为什么选A,而不选其他几项,则要弄清选项中含way的四个短语的不同意义和用法,下面我们就对此作一归纳和小结。 一、in a way的用法 表示:在一定程度上,从某方面说。如: In a way he was right.在某种程度上他是对的。注:in a way也可说成in one way。 二、on the way的用法 1、表示:即将来(去),就要来(去)。如: Spring is on the way.春天快到了。 I'd better be on my way soon.我最好还是快点儿走。 Radio forecasts said a sixth-grade wind was on the way.无线电预报说将有六级大风。 2、表示:在路上,在行进中。如: He stopped for breakfast on the way.他中途停下吃早点。 We had some good laughs on the way.我们在路上好好笑了一阵子。 3、表示:(婴儿)尚未出生。如: She has two children with another one on the way.她有两个孩子,现在还怀着一个。 She's got five children,and another one is on the way.她已经有5个孩子了,另一个又快生了。 三、by the way的用法
英语比较级和最高级
形容词比较级和最高级的形式 一、形容词比较级和最高级的构成 形容词的比较级和最高级变化形式规则如下 构成法原级比较级最高级 ①一般单音节词末尾加 er 和 est strong stronger strongest ②单音节词如果以 e结尾,只加 r 和 st strange stranger strangest ③闭音节单音节词如末尾只有一个辅音字母, 须先双写这个辅音字母,再加 er和 est sad big hot sadder bigger hotter saddest biggest hottest ④少数以 y, er(或 ure), ow, ble结尾的双音节词, 末尾加 er和 est(以 y结尾的词,如 y前是辅音字母, 把y变成i,再加 er和 est,以 e结尾的词仍 只加 r和 st) angry Clever Narrow Noble angrier Cleverer narrower nobler angriest cleverest narrowest noblest ⑤其他双音节和多音节词都在前面加单词more和most different more different most different 1) The most high 〔A〕mountain in 〔B〕the world is Mount Everest,which is situated 〔C〕in Nepal and is twenty nine thousand one hundred and fourty one feet high 〔D〕 . 2) This house is spaciouser 〔A〕than that 〔B〕white 〔C〕one I bought in Rapid City,South Dakota 〔D〕last year. 3) Research in the social 〔A〕sciences often proves difficulter 〔B〕than similar 〔C〕work in the physical 〔D〕sciences. 二、形容词比较级或最高级的特殊形式:
高中英语的比较级和最高级用法总结
比较级和最高级 1.在形容词词尾加上―er‖ ―est‖ 构成比较级、最高级: bright(明亮的)—brighter—brightest broad(广阔的)—broader—broadest cheap(便宜的)—cheaper—cheapest clean(干净的)—cleaner—cleanest clever(聪明的)—cleverer—cleverest cold(寒冷的)—colder—coldest cool(凉的)—cooler—coolest dark(黑暗的)—darker—darkest dear(贵的)—dearer—dearest deep(深的)—deeper—deepest fast(迅速的)—faster—fastest few(少的)—fewer—fewest great(伟大的)—greater—greatest hard(困难的,硬的)—harder—hardest high(高的)—higher—highest kind(善良的)—kinder—kindest light(轻的)—lighter—lightest long(长的)—longer—longest loud(响亮的)—louder—loudest low(低的)—lower—lowest near(近的)—nearer—nearest new(新的)—newer—newest poor(穷的)—poorer—poorest quick(快的)—quicker—quickest quiet(安静的)—quieter—quietest rich(富裕的)—richer—richest short(短的)—shorter—shortest slow(慢的)—slower—slowest small(小的)—smaller—smallest smart(聪明的)—smarter—smartest soft(柔软的)—softer—softest strong(强壮的)—stronger—strongest sweet(甜的)—sweeter—sweetest tall(高的)-taller-tallest thick(厚的)—thicker—thickest warm(温暖的)—warmer—warmest weak(弱的)—weaker—weakest young(年轻的)—younger—youngest 2.双写最后一个字母,再加上―er‖ ―est‖构成比较级、最高级: big(大的)—bigger—biggest fat(胖的)—fatter—fattest hot(热的)—hotter—hottest red(红的)—redder—reddest sad(伤心的)—sadder—saddest thin(瘦的)—thinner—thinnest wet(湿的)—wetter—wettest mad(疯的)—madder—maddest 3.以不发音的字母e结尾的形容词,加上―r‖ ―st‖ 构成比较级、最高级:able(能干的)—abler—ablest brave(勇敢的)—braver—bravest close(接近的)—closer—closest fine(好的,完美的)—finer—finest large(巨大的)—larger—largest late(迟的)—later—latest nice(好的)—nicer—nicest ripe(成熟的)—riper—ripest
The way的用法及其含义(一)
The way的用法及其含义(一) 有这样一个句子:In 1770 the room was completed the way she wanted. 1770年,这间琥珀屋按照她的要求完成了。 the way在句中的语法作用是什么?其意义如何?在阅读时,学生经常会碰到一些含有the way 的句子,如:No one knows the way he invented the machine. He did not do the experiment the way his teacher told him.等等。他们对the way 的用法和含义比较模糊。在这几个句子中,the way之后的部分都是定语从句。第一句的意思是,“没人知道他是怎样发明这台机器的。”the way的意思相当于how;第二句的意思是,“他没有按照老师说的那样做实验。”the way 的意思相当于as。在In 1770 the room was completed the way she wanted.这句话中,the way也是as的含义。随着现代英语的发展,the way的用法已越来越普遍了。下面,我们从the way的语法作用和意义等方面做一考查和分析: 一、the way作先行词,后接定语从句 以下3种表达都是正确的。例如:“我喜欢她笑的样子。” 1. the way+ in which +从句 I like the way in which she smiles. 2. the way+ that +从句 I like the way that she smiles. 3. the way + 从句(省略了in which或that) I like the way she smiles. 又如:“火灾如何发生的,有好几种说法。” 1. There were several theories about the way in which the fire started. 2. There were several theories about the way that the fire started.
(完整版)初中英语比较级和最高级的用法
英语语法---比较级和最高级的用法 在英语中通常用下列方式表示的词:在形容词或副词前加more(如 more natural,more clearly )或加后缀 -er(newer,sooner )。典型的是指形容词或副词所表示的质、量或关系的增加。英语句子中,将比较两个主体的方法叫做“比较句型”。其中,像“A比B更……”的表达方式称为比较级;而“A最……”的表达方式则称为最高级。组成句子的方式是将形容词或副词变化成比较级或最高级的形态。 一、形容词、副词的比较级和最高级的构成规则 1.一般单音节词和少数以-er,-ow结尾的双音节词,比较级在后面加-er,最高级在后面加-est; (1)单音节词 如:small→smaller→smallest short→shorter→shortest tall→taller→tallest great→greater→greatest (2)双音节词 如:clever→cleverer→cleverest narrow→narrower→narrowest 2.以不发音e结尾的单音节词,比较在原级后加-r,最高级在原级后加-st; 如:large→larger→largest nice→nicer→nicest able→abler→ablest 3.在重读闭音节(即:辅音+元音+辅音)中,先双写末尾的辅音字母,比较级加-er,最高级加-est; 如:big→bigger→biggest hot→hotter→hottest fat→fatter→fattest 4.以“辅音字母+y”结尾的双音节词,把y改为i,比较级加-er,最高级加-est; 如:easy→easier→easiest heavy→heavier→heaviest busy→busier→busiest happy→happier→happiest 5.其他双音节词和多音节词,比较级在前面加more,最高级在前面加most; 如:beautiful→more beautiful→most beautiful different→more different→most different easily→more easily→most easily
