原子掺杂改善石墨相氮化碳光催化性能的 研究进展

Journal of Advances in Physical Chemistry 物理化学进展, 2017, 6(2), 84-96
Published Online May 2017 in Hans. https://www.360docs.net/doc/1b4275077.html,/journal/japc
https://https://www.360docs.net/doc/1b4275077.html,/10.12677/japc.2017.62011
Research Progress on Improving the
Photocatalysis of Graphite-C3N4 via
O, S and P Doping
Ran You, Lu Chen, Yongping Zhang
Faculty of Materials and Energy, Southwest University, Chongqing
Received: May 4th, 2017; accepted: May 21st, 2017; published: May 24th, 2017
Abstract
Graphitic arbon nitride (g-C3N4) has attracted extensive attention in the field of photocatalysis because of its unique atomic and electronic structures. Some intrinsic characteristics, such as small specific surface area and rapid recombination of the photogenerated electron-hole pair, re-strict its application. It is a valid pathway to improve the photocatalytic performance of g-C3N4 by changing its physical and chemical properties via atomic doping. In this paper, we overview the ways to optimize the photocatalysis of g-C3N4, and focus on the techniques and effect of atomic doping. Finally, the research perspective on the g-C3N4 is discussed.
Keywords
Template, Graphitic-C3N4, Atomic Doping, Photocatalytic Performance
原子掺杂改善石墨相氮化碳光催化性能的
研究进展
游然,陈露,张永平
西南大学材料与能源学部,重庆
收稿日期:2017年5月4日;录用日期:2017年5月21日;发布日期:2017年5月24日
摘要
石墨相氮化碳(g-C3N4)独特的结构使其在光催化性能上具有一定的优越性,使其研究受到了广泛的关注。
文章引用: 游然, 陈露, 张永平. 原子掺杂改善石墨相氮化碳光催化性能的研究进展[J]. 物理化学进展,2017, 6(2):
游然 等
但g-C 3N 4较低的比表面积以及较高的光生载流子复合率限制了其应用,为了进一步改善g-C 3N 4的性能,通过原子和分子掺杂改变它的电子结构来改变其物理和化学性质是一个有效途径。本文就如何优化g-C 3N 4的光催化性能做了简单的概述,重点介绍了非金属原子O, S, P 掺杂对g-C 3N 4性能的影响,并展望了未来g-C 3N 4研究的动向。
关键词
模板,g-C 3N 4,原子掺杂,光催化性能
Copyright ? 2017 by authors and Hans Publishers Inc. This work is licensed under the Creative Commons Attribution International License (CC BY).
https://www.360docs.net/doc/1b4275077.html,/licenses/by/4.0/
1. 引言
人类社会的加速发展使得能源短缺和环境污染问题越来越严重。如何利用光催化剂将太阳能转换为可直接利用的能源,或者使污染物降解是当今研究的热点之一。人们关注的一些光催化剂TiO 2、ZnO 、BiWO 6等半导体材料在结构上或反应过程中存在一定缺陷,如,CB(导带)位置相对较低,使得其光生电子的还原能力较弱[1] [2];或能带间隙较大,使其对可见光的吸收能力有限[3];或会随着反应过程中贵金属的沉积表现出不稳定状态[4] [5] [6]。石墨型C 3N 4(g-C 3N 4)作为一种具有可见光响应的非金属半导体材料[7],具有独特的材料结构和电子结构,在性能上具有一定的优越性。
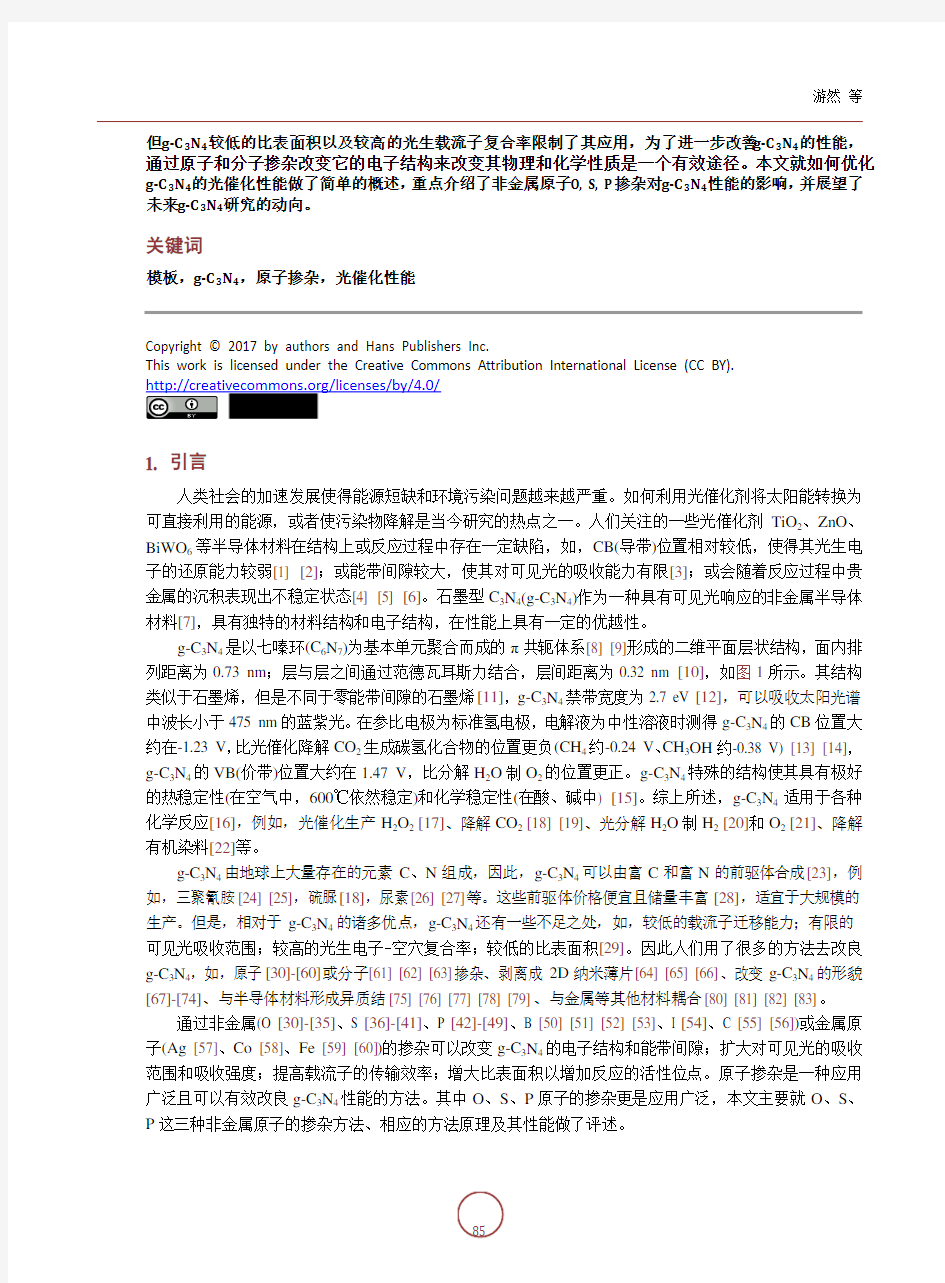
g-C 3N 4是以七嗪环(C 6N 7)为基本单元聚合而成的π共轭体系[8] [9]形成的二维平面层状结构,面内排列距离为0.73 nm ;层与层之间通过范德瓦耳斯力结合,层间距离为0.32 nm [10],如图1所示。其结构类似于石墨烯,但是不同于零能带间隙的石墨烯[11],g-C 3N 4禁带宽度为2.7 eV [12],可以吸收太阳光谱中波长小于475 nm 的蓝紫光。在参比电极为标准氢电极,电解液为中性溶液时测得g-C 3N 4的CB 位置大
约在-1.23 V ,比光催化降解CO 2生成碳氢化合物的位置更负(CH 4约-0.24 V 、
CH 3OH 约-0.38 V) [13] [14],g-C 3N 4的VB(价带)位置大约在1.47 V ,比分解H 2O 制O 2的位置更正。g-C 3N 4特殊的结构使其具有极好的热稳定性(在空气中,600℃依然稳定)和化学稳定性(在酸、碱中) [15]。综上所述,g-C 3N 4适用于各种化学反应[16],例如,光催化生产H 2O 2 [17]、降解CO 2 [18] [19]、光分解H 2O 制H 2 [20]和O 2 [21]、降解有机染料[22]等。
g-C 3N 4由地球上大量存在的元素C 、N 组成,因此,g-C 3N 4可以由富C 和富N 的前驱体合成[23],例如,三聚氰胺[24] [25],硫脲[18],尿素[26] [27]等。这些前驱体价格便宜且储量丰富[28],适宜于大规模的生产。但是,相对于g-C 3N 4的诸多优点,g-C 3N 4还有一些不足之处,如,较低的载流子迁移能力;有限的可见光吸收范围;较高的光生电子-空穴复合率;较低的比表面积[29]。因此人们用了很多的方法去改良g-C 3N 4,如,原子[30]-[60]或分子[61] [62] [63]掺杂、剥离成2D 纳米薄片[64] [65] [66]、改变g-C 3N 4的形貌
[67]-[74]、与半导体材料形成异质结[75] [76] [77] [78] [79]、与金属等其他材料耦合[80] [81] [82] [83]。
通过非金属(O [30]-[35]、S [36]-[41]、P [42]-[49]、B [50] [51] [52] [53]、I [54]、C [55] [56])或金属原子(Ag [57]、Co [58]、Fe [59] [60])的掺杂可以改变g-C 3N 4的电子结构和能带间隙;扩大对可见光的吸收范围和吸收强度;提高载流子的传输效率;增大比表面积以增加反应的活性位点。原子掺杂是一种应用广泛且可以有效改良g-C 3N 4性能的方法。其中O 、S 、P 原子的掺杂更是应用广泛,本文主要就O 、S 、P 这三种非金属原子的掺杂方法、相应的方法原理及其性能做了评述。
Open Access
游然等
(a)
(b)
Figure 1. (a) planar and (b) interlayer structural illustration of g-C3N4 (C, N indicated by gray, blue spheres, respectively). A heptazine unit (C6N7) is highlighted in yellow [10]
图1. g-C3N4平面(a)及层间(b)结构示意图(C, N原子分别用灰色,蓝色圆球表示), 七嗪环(C6N7) 单元用黄色标出[10]
2. O原子的掺杂
众所周知,g-C3N4和石墨烯拥有相似的结构以及化学性质,而石墨烯的光电性质与石墨烯的含O水平有极其紧密的联系[84]。因此,人们猜想g-C3N4的性能是否也与其含O量有关[35]。O原子的电负性是3.44 eV,N原子的电负性是3.04 eV。O原子相对较高的电负性有利于限制电子的移动[34]。因此,N-O、C-O、C=O这些含O官能团可作为吸电子集团,在光催化反应中促进电子-空穴的分离这方面扮演着很重要的角色。
2.1. 水热法
水热法是形成含O官能团,进行O掺杂的常用手段。常以H2O2作为反应溶剂[33] [34] [35]。Li等人
[33]以H2O2作为反应溶剂,在反应釜中水热合成O-g-C3N4(O原子掺杂的g-C3N4),实验测得其降解亚甲
基蓝的速率是体相g-C3N4的4倍,制H2速率是其2.5倍。
Ming等人[35]将制得的g-C3N4在180℃的条件下,分别水热不同时间,发现在水热12 h时,g-C3N4的含O量达到最大,继续水热,含O量基本不变。且水热12 h所得g-C3N4的光载流子响应可以达到体相g-C3N4的10倍,光催化降解AO7 (酸性橙7)的速率可以达到其7倍。
2.2. 超分子自组装
水超分子自组装是利用分子间氢键的方向性合成在结构上高度有序的分子聚集体的合成过程。其高
游然等
度有序的结构可以减少缺陷的产生,而缺陷(g-C3N4在脱氨基过程中产生的NH/NH2组织)通常会成为光生电子-空穴的复合中心,因而超分子合成有利于光生电子-空穴的分离,从而促进g-C3N4光电性能的提高。
Huang等人[31]用H2O2前处理三聚氰胺,使其在氢键作用下形成凝胶状物质,该过程为三聚氰胺的超分子自组装过程。然后,将所得凝胶状物质离心,干燥,研磨。再将所得的粉末状物质以5℃/min的速率加热到550℃并保温2 h,得到多孔且掺O的g-C3N4。如图2所示为前处理成过程。这种前处理方法简单,但是可以直接合成既有一定形貌特点又掺杂O原子的g-C3N4,为原子掺杂与形貌改良共进的多重优化方式提供了参考价值,这种方法有效改善了g-C3N4的光电性能。
2.3. 芬顿反应
水H2O2与二价铁离子Fe2+的混合溶液为芬顿试剂,具有很强的氧化性,可以将很多的有机化合物氧化为无机态。Guo等人[30]将合成的体相g-C3N4用酸刻蚀剥离形成g-C3N4薄片,然后,再用芬顿试剂处理,芬顿反应如图3所示。芬顿试剂的强氧化性可以使原先剥离出来的g-C3N4薄片上的缺陷位点氧化形成孔隙,还可以使O原子掺入到g-C3N4薄片的边缘,形成含有多孔结构且边缘掺O的g-C3N4薄片。
水在对g-C3N4进行O原子掺杂的过程中人们发现,O原子的掺入确实可以促进电子-空穴的分离,减小g-C3N4的能带间隙,提高g-C3N4对可见光的吸光范围。
3. S原子的掺杂
阴离子掺杂是一种可以有效提高g-C3N4可见光吸收范围的方法[38] [39]。另外,掺杂具有较高元素周期序数的元素也可以降低g-C3N4的能带间隙,从而扩大对可见光的吸收范围[41]。S元素既具有较高的元素周期序数,同时又可作为阴离子掺杂改良g-C3N4,因此受到了人们的关注。
Figure 2. Illustration of fabrication of g-C3N4 with porous network and O-doping by hydrogen bond-induced supramolecular assembly (C, N and substitutional O are indicated by gray, blue and red spheres, respectively [31]
图2. (a)超分子自组装合成O原子掺杂且多孔g-C3N4的示意图(灰、蓝、红色的小球分别代表C、N、O原子) [31]
Figure 3. Schematic illustration of the O-g-C3N4 thin sheets formed via photo-Fenton reaction [30]
图3. 芬顿反应合成O-g-C3N4薄片示意图[30]
游然等
根据S源的性质,我们把掺S的方法分为两大类,1、以H2S气体为S源,在S源的气体氛围下合成S-g-C3N4(S原子掺杂的g-C3N4)。2、分别以TU(硫脲)、TCA(三聚硫氰酸)为S源自合成S-g-C3N4。
3.1. S源作为气体氛围
比结构中替代原子具有更低电负性的原子的匀质掺杂可以增加VB的宽度。VB的宽度影响并控制空穴的移动。VB的宽度越宽,空穴的移动性越强,其氧化作用越强。但是,原子的匀质掺杂因为其有限的溶解度而很难实现。
Liu等人[39]以双氰胺为前驱体,合成g-C3N4,研磨后置于H2S气体(纯度,99.99%,通气速率,12 ml/min)的氛围下,升温到450℃再保温1 h,合成S-g-C3N4。通过长时间的Ar+刻蚀发现S的2p峰并没有出现变化,证明在H2S气体的氛围下,实现了对S的匀质掺杂。S掺杂的g-C3N4在λ > 400 nm时仍然可以完全氧化苯酚,但是这个氧化过程对于未掺杂的g-C3N4来说,在λ > 300 nm时已然不可能发生。说明S的匀质掺杂确实扩大了对可见光的响应范围,增强了氧化性。
3.2. 自合成
不同于以S源做为气体氛围时需要先制备Bulk-g-C3N4(体相g-C3N4),以TCA [37] [41]和TU [36] [38]
[40]做为前驱体可以直接合成S-g-C3N4。
以富C、N材料(如,氨腈、双氰胺、三聚氰胺)作为前驱体时,由于动力学的原因,聚合并不完全[10]。
而由于不完全聚合产生的缺陷(如,NH/NH2组织)可作为光生电子-空穴的复合中心,降低载流子的浓度,从而减小g-C3N4的活性[85]。
Zhang等人[41]以TCA为前驱体,自合成了S-g-C3N4。合成过程中-SH代替原先的氨基组织,成为新的游离基,促进了g-C3N4的聚合。实验证明新合成的S-g-C3N4的制H2能力是传统未掺杂g-C3N4的14倍。通常,分解H2O制O2很难通过单一的催化剂催化实现,但是,该方法合成的g-C3N4制O2能力是传统g-C3N4的5倍,因此,其可以在没有酸辅助的作用下催化H2O制O2,且能达到可观的速率。
Hong等人[38]以TU为前驱体,自合成了S-g-C3N4,其合成机理如图4。Hong等人认为TU在热聚合的过程中有中间产物(氨腈、三聚氰胺、三聚氰酸等)生成,中间产物TCA的-SH和中间产物氨腈或三聚氰胺的-NH作用,促进S原子进入g-C3N4的结构中。经试验测试得到,用硫脲自合成得到的S-g-C3N4的制H2能力可以达到g-C3N4的10倍。
Figure 4. Proposed formation mechanism of sulfur-doped g-C3N4 from condensation of thiourea [38]
图4. 硫脲合成S掺杂g-C3N4的过程示意图[38]
游然等4. P原子掺杂
P原子的掺入可以抑制晶体的生长,扩大g-C3N4的比表面积,为催化反应的进行提供更多的反应活性位点[86];还可以改良g-C3N4的电子结构[45] [49]。如图5所示,当P原子取代C位后,P原子3/5的价电子与N形成P-N共价键,剩余的孤电子对参与到三嗪环的π共轭离域体系,剩下P+成为路易斯酸位点,促进光生电子和空穴的分离;另外,g-C3N4上的路易斯碱位点(-NH2)和路易斯酸位点(P+)构成的酸碱双功能团有助于实现对环氧衍生物的CO2环加成[44]。现在我们就P的掺杂方法进行了总结。
4.1. 简单混合热聚合
简单混合热聚合是指将P源和生成g-C3N4的前驱体进行简单混合,再加热保温处理的过程。其方法简单,温和,但合成的P-g-C3N4(P原子掺杂的g-C3N4)的活性受到P源的影响。
离子液体通常指熔化温度小于100℃的有机盐液体,其全部由离子组成。离子液体具有较高的化学稳定性和热稳定性,因而被广泛应用在很多的领域[87]。
Zhang和他的团队[48]以离子液体BmimBF6和双氰胺作为前驱体,合成了P-g-C3N4。在反应过程中,BmimBF6因具有较高的热稳定性,不会在双氰胺热聚合之前发生分解,但随着温度的继续升高,PF6-与氨基集团作用,使P原子成功掺入到C-N的结构。之后,Zhang、Chen等人[47]在以相同的P源和前驱体合成的P-g-C3N4中发现了多孔结构,其认为BmimBF6不仅作为P源参与反应而且在聚合过程中充当了软模板的角色,产生了碳氢化合物和NH4F等可以分解产生气体的物质。
用离子溶液作为P源方法简单,但其价格昂贵,且掺P量较少,对g-C3N4性能的改善有限。所以Hu等人[43]将P源改为(NH4)2HPO4,制得P-g-C3N4。同时,作为对比,以相同质量比的离子液体BmimBF6作为P源,用相同方法制得P-g-C3N4,记为IL- P-g-C3N4,研究它们制氢能力。发现P-g-C3N4的制氢能力是IL-P-g-C3N4的1.8倍。
如图6(a)、图6(b)所示,是分别以BmimBF6、(NH4)2HPO4为P源的P原子的两种可能的掺杂位置。不同的P源会影响P原子在g-C3N4结构中的掺杂位置以及掺杂量,从而导致不同的催化能力[43]。所以人们致力于寻求合适的P源,来获得性能理想的P-g-C3N4。
Figure 5. The electronic structure and the possible doping sites of P-g-C3N4. [49]
图5. P-g-C3N4的电子结构及可能的P原子的掺杂位置[49]
游然等
(a) (b)
Figure 6. The possible doping sites of P atoms in g-C3N4 by using (a) BmimBF6 and (b)(NH4)2HPO4 as precursor [43]
图6. (a) BmimBF6为前驱体时P原子可能掺杂的位置;(b) (NH4)2HPO4为前驱体时P原子可能掺杂的位置[43]
Zhou等人[49]和Lan [44]等人将HCCP(六氯环三磷腈)作为P源,HCCP的价格要优于BmimBF6,同时,HCCP拥有类三嗪的P-N环结构与g-C3N4的结构非常吻合,且P-Cl键非常活泼,可以在聚合过程中与-NH2发生反应,促进脱氨基过程的进行,降低反应温度以及引入P原子。图5所示为以HCCP作为P 源时,P原子的掺杂位置示意图。实验显示以HCCP作为P源合成的P-g-C3N4降解有机染料罗丹明的速率是一般g-C3N4的3倍,制氢速率是一般g-C3N4的2.9倍[49],另外,在对环氧衍生物加成CO2也有出色的催化效果[44]。
4.2. 酸-碱作用
P源和生成g-C3N4的前驱体之间不再只是简单的混合,它们之间会因为酸碱作用,紧密地交联在一起。Ran等人[46]用三聚氰胺和AEP(2-氨乙基磷酸)合成了多孔的P-g-C3N4。合成示意图如图7所示。三聚氰胺与AEP之间的酸碱作用和范德瓦耳斯力促进了均匀介孔结构的产生,使P-g-C3N4具有较大的比表面积,有利于增加其催化能力。
Ma等人[45]用碳纤维纸、三聚氰胺、乙二磷酸为先驱体合成了花状结构的P-g-C3N4,其合成示意图如图8所示。碳纤维纸、三聚氰胺、乙二磷酸之间的酸碱作用,促进前驱体吸附在碳纤维纸上,有利于P-g-C3N4花状纳米结构的生长及P-g-C3N4与碳纤维纸的耦合。
总之,P源和生成g-C3N4的前驱体之间的酸碱作用力在促进P原子引入结构的同时,有助于其他类似介孔的匀质结构及可控形貌的生成,从而在掺杂与形貌的多作用力下增强P-g-C3N4的催化能力。
4.3. 超分子自组装
与O原子掺杂时所用的方法类似,P原子的掺杂也可以通过超分子自组装来实现,所用溶剂除了在提供掺杂原子上有所区别外,也会影响g-C3N4结构的变化,不同溶剂会合成不同形貌的g-C3N4[88]。
Guo和他的团队[42]以三聚氰胺和H3PO4作为反应的原材料合成了具有六方棒状的P-g-C3N4。其中H3PO4水溶液的作用有三,1、H3PO4提供了一定的酸性环境,辅助水解三聚氰胺形成三聚氰酸。2、H3PO4作为P源合成P-g-C3N4。3、作为超分子自组装的溶剂。有文献研究表明,作为g-C3N4前驱体的三聚氰胺可以和三嗪衍生物,如,三聚氰酸,通过氢键形成具有特定形貌的超分子结构[88] [89] [90]。在Guo 的实验中,三聚氰胺和经H3PO4辅助水解得到的三聚氰酸自组装合成具有六方棒状的超分子前驱体——MCA,H3PO4分子吸附在MCA的表面,再经过N2作为保护气体的热聚合反应后,P原子进入g-C3N4的结构中。具体的合成过程可参见图9。
游然等
Figure 7. Synthesis procedure of porous P-g-C3N4 using acid-base interaction [46]
图7. 通过酸-碱作用合成多孔P-g-C3N4示意图[46]
Figure 8. Fabrication of P-g-C3N4 nanostructures directly grown on CFP usingacid-base interaction. [45]
图8. 通过酸碱作用在CFP上合成纳米结构的P-g-C3N4。[45]
Figure 9. The formation process of phosphorus-doped tubular carbon nitride by supramolecular assembly [42]
图9. 通过合成过超分子自组装原理合成P-g-C3N4的管状结构[42]
游然等
该研究通过对前驱体的处理,在引入掺杂原子的同时,自组装改变了g-C3N4的形貌,是实现多重优化的有利手段。
5. 双原子掺杂
单一的原子掺杂已经对g-C3N4改性起到了有效作用,双原子掺杂可以协同影响g-C3N4的性能,使其具有更好的光催化效果。
You等人[91]以三聚硫氰酸为S源,以过氧化氢为O源合成了S,O原子双掺杂的g-C3N4,研究表明,单掺S原子的g-C3N4光催化性能是纯的体相g-C3N4的1.5倍,但双原子掺杂后其光催化性能为纯的体相g-C3N4的6倍。显然,双原子掺杂起到了很好的协同作用,有效改善了g-C3N4的光催化性能。
6. 结论与展望
g-C3N4是一种类似于石墨烯的层状非金属半导体材料,具有独特的电子结构和极好的热稳定性、化学稳定性和良好的光催化性能,因而受到了科学家的关注。但是g-C3N4的性能却受制于其有限可见光的吸收范围、较小的比表面积及较快的光电子-空穴复合率。改良g-C3N4光催化性能有多种手段,原子掺杂是其中应用最为广泛的,同时,也是最有效的手段之一。单一的原子掺杂就可以实现对g-C3N4性能的有效改善,而利用双原子掺杂或者利用超分子自组装,分子间酸-碱作用力这样的方法引入掺杂原子,同时还可以实现掺杂原子协同作用或掺杂与形貌结构共进的多重优化,有利于促进g-C3N4性能的改善,可能成为今后g-C3N4改性研究的发展方向。
资助信息
国家自然科学基金(批准号,21173170);中央高校基本科研业务费资助项目(XDJK2016D002)。
参考文献(References)
[1]Chen, X., Zhou, Y., Liu, Q., et al. (2012) Ultrathin Single-Crystal WO3 Nanosheets by Two-Dimensional Oriented At-
tachment toward Enhanced Photocatalystic Reduction of CO2 into Hydrocarbon Fuels under Visible Light. ACS Ap-
plied Materials & Interfaces, 4, 3372-3377. https://https://www.360docs.net/doc/1b4275077.html,/10.1021/am300661s
[2]Zhou, Y., Tian, Z., Zhao, Z., et al. (2011) High-Yield Synthesis of Ultrathin and Uniform Bi2WO6 Square Nanoplates
Benefitting from Photocatalytic Reduction of CO2 Intorenewable Hydrocarbon Fuel under Visible Light. ACS Applied
Materials & Interfaces, 3, 3594-3601. https://https://www.360docs.net/doc/1b4275077.html,/10.1021/am2008147
[3]Xi, G., Ouyang, S. and Ye, J. (2011) General Synthesis of Hybrid TiO2 Mesoporous “French Fries” toward Improved
Photocatalytic Conversion of CO2 into Hydrocarbon Fuel, a Case of TiO2/ZnO. Chemistry, 17, 9057-9061.
[4]He, Y., Zhang, L., Teng, B., et al. (2015) New Application of Z-Scheme Ag3PO4/G-C3N4 Composite in Converting
CO2 to Fuel. Environmental Science & Technology, 49, 649-656. https://https://www.360docs.net/doc/1b4275077.html,/10.1021/es5046309
[5]Hu, J.S., Ren, L.L., Guo, Y.G., et al. (2005) Mass Production and High Photocatalytic Activity of ZNS Nanoporous
Nanoparticles. Angewandte Chemie International Edition in English, 44, 1269-1273.
[6]Li, Q., Guo, B., Yu, J., et al. (2011) Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production of
CdS-Cluster-Decorated Graphene Nanosheets. Journal of the American Chemical Society, 133, 10878-10884.
https://https://www.360docs.net/doc/1b4275077.html,/10.1021/ja2025454
[7]Shalom, M., Gimenez, S., Schipper, F., et al. (2014) Controlled Carbon Nitride Growth on Surfaces for Hydrogen
Evolution Electrodes. Angewandte Chemie International Edition in English, 53, 3654-3658.
https://https://www.360docs.net/doc/1b4275077.html,/10.1002/anie.201309415
[8]Chen, L., Huang, D., Ren, S., et al. (2013) Preparation of Graphite-Like Carbon Nitride Nanoflake Film with Strong
Fluorescent and Electrochemiluminescent Activity. Nanoscale, 5, 225-230. https://https://www.360docs.net/doc/1b4275077.html,/10.1039/C2NR32248J
[9]Gong, Y., Li, M., Li, H., et al. (2015) Graphitic Carbon Nitride Polymers, Promising Catalysts or Catalyst Supports for
Heterogeneous Oxidation and Hydrogenation. Green Chemistry, 17, 715-736. https://https://www.360docs.net/doc/1b4275077.html,/10.1039/C4GC01847H
[10]Bojdys, M.J., Muller, J.O., Antonietti, M., et al. (2008) Ionothermal Synthesis of Crystalline, Condensed, Graphitic
Carbon Nitride. European Journal of Inorganic Chemistry, 14, 8177-8182. https://https://www.360docs.net/doc/1b4275077.html,/10.1002/chem.200800190
游然等[11]Castro, E.V., Novoselov, K.S., Morozov, S.V., et al. (2007) Biased Bilayer Graphene, Semiconductor with a Gap
Tunable by the Electric Field Effect. European Journal of Inorganic Chemistry, 99, Article ID: 216802.
https://https://www.360docs.net/doc/1b4275077.html,/10.1103/physrevlett.99.216802
[12]Wang, X., Maeda, K., Thomas, A., et al. (2009) A Metal-Free Polymeric Photocatalyst for Hydrogen Production from
Water under Visible Light. Nature Materials, 8, 76-80. https://https://www.360docs.net/doc/1b4275077.html,/10.1038/nmat2317
[13]Wang, C.J., Thompson, R.L, Baltrus, J., et al. (2010) Visible Light Photoreduction of CO2 Using CdSe/Pt/TiO2 Hete-
rostructured Catalysts. The Journal of Physical Chemistry Letters, 1, 48-53. https://https://www.360docs.net/doc/1b4275077.html,/10.1021/jz9000032
[14]Marszewski, M., Cao, S., Yu, J., et al. (2015) Semiconductor-Based Photocatalytic CO2 Conversion. Materials Hori-
zons, 2, 261-278. https://https://www.360docs.net/doc/1b4275077.html,/10.1039/C4MH00176A
[15]Zhu, J., Xiao, P., Li, H., et al. (2014) Graphitic Carbon Nitride, Synthesis, Properties and Applications in Catalysis.
ACS Applied Materials & Interfaces, 6, 16449-16465.
[16]Thomas, A., Fischer, A., Goettmann, F., et al. (2008) Graphitic Carbon Nitride Materials, Variation of Structure and
Morphology and Their Use as Metal-Free Catalysts. Journal of Materials Chemistry, 18, 4893-4908.
https://https://www.360docs.net/doc/1b4275077.html,/10.1039/b800274f
[17]Li, S., Dong, G., Hailili, R., et al. (2016) Effective Photocatalytic H2O2 Production under Visible Light Irradiation at
G-C3N4 Modulated by Carbon Vacancies. Applied Catalysis B, 190, 26-35.
https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.apcatb.2016.03.004
[18]Wang, H., Sun, Z., Li, Q., et al. (2016) Surprisingly Advanced CO2 Photocatalytic Conversion over Thiourea Derived
G-C3N4 with Water Vapor while Introducing 200-420nm UV Light. Journal of CO2 Utilization, 14, 143-151.
https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.jcou.2016.04.006
[19]Li, M., Zhang, L., Fan, X., et al. (2015) Highly Selective CO2 Photoreduction to CO over G-C3N4/Bi2WO6 Composites
under Visible Light. Journal of Materials Chemistry, 3, 5189-5196.
[20]Martín-Ramos, P., Martín-Gil, J., Dante, R.C., et al. (2015) A Simple Approach to Synthesize G-C3N4 with High Visi-
ble Light Photoactivity for Hydrogen Production. International Journal of Hydrogen Energy, 40, 7273-7281.
[21]Li, Z., Kong, C. and Lu, G. (2016) Visible Photocatalytic Water Splitting and Photocatalytic Two-Electron Oxygen
Formation over Cu-and Fe-Doped G-C3N4. The Journal of Physical Chemistry C, 120, 56-63.
https://https://www.360docs.net/doc/1b4275077.html,/10.1021/acs.jpcc.5b09469
[22]Bhowmik, T., Kundu, M.K. and Barman, S. (2015) Ultra Small Gold Nanoparticles-Graphitic Carbon Nitride Compo-
site, an Efficient Catalyst for Ultrafast Reduction of 4-Nitrophenol and Removal of Organic Dyes from Water. RSC Advances, 5, 38760-38773.
[23]Cao, S., Low, J., Yu, J., et al. (2015) Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Advanced Materials,
27, 2150-2176.
[24]Yan, S.C., Li, Z.S. and Zou, Z.G. (2009) Photodegradation Performance of G-C3N4Fabricated by Directly Heating
Melamine. Langmuir, 25, 10397-10401. https://https://www.360docs.net/doc/1b4275077.html,/10.1021/la900923z
[25]Komatsu, T. (2001) Attempted Chemical Synthesis of Graphite-Like Carbon Nitride. Journal of Materials Chemistry,
11, 799-801.
[26]Martin, D.J., Qiu, K.P., Shevlin, S.A., et al. (2014) Highly Efficient Photocatalytic H2 Evolution from Water Using
Visible Light and Structure-Controlled Graphitic Carbon Nitride. Angewandte Chemie International Edition, 53, 9240-9245. https://https://www.360docs.net/doc/1b4275077.html,/10.1002/anie.201403375
[27]Zhang, Y., Liu, J., Wu, G., et al. (2012) Porous Graphitic Carbon Nitride Synthesized via Direct Polymerization of
Urea for Efficient Sunlight-Driven Photocatalytic Hydrogen Production. Nanoscale, 4, 5300-5303.
[28]Fina, F., Menard, H. and Irvine, J.T. (2015) The Effect of Pt NPs Crystallinity and Distribution on the Photocatalytic
Activity of Pt-G-C3N4. Physical Chemistry Chemical Physics, 17, 13929-13936. https://https://www.360docs.net/doc/1b4275077.html,/10.1039/C5CP00560D [29]Shi, H., Chen, G., Zhang, C., et al. (2014) Polymeric G-C3N4Coupled with NaNbO3Nanowires toward Enhanced
Photocatalytic Reduction of CO2 into Renewable Fuel. ACS Catalysis, 4, 3637-3643.
[30]Guo, S., Zhu, Y., Yan, Y., et al. (2016) Holey Structured Graphitic Carbon Nitride Thin Sheets with Edge Oxygen
Doping via Photo-Fenton Reaction with Enhanced Photocatalytic Activity. Applied Catalysis B: Environmental, 185, 315-321. https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.apcatb.2015.11.030
[31]Huang, Z.F., Song, J., Pan, L., et al. (2015) Carbon Nitride with Simultaneous Porous Network and O-Doping for Effi-
cient Solar-Energy-Driven Hydrogen Evolution. Nano Energy, 12, 646-656.
[32]Kharlamov, A., Bondarenko, M. and Kharlamova, G. (2016) Method for the Synthesis of Water-Soluble Oxide of
Graphite-Like Carbon Nitride. Diamond and Related Materials, 61, 46-55.
https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.diamond.2015.11.006
[33]Li, J., Shen, B., Hong, Z., et al. (2012) A Facile Approach to Synthesize Novel Oxygen-Doped G-C3n4 with Superior
游然等
Visible-Light Photoreactivity. Chemical communications, 48, 12017-12019.
[34]Liu, S., Li, D., Sun, H., et al. (2016) Oxygen Functional Groups in Graphitic Carbon Nitride for Enhanced Photocata-
lysis. Journal of Colloid and Interface Science, 468, 176-182.
[35]Ming, L., Yue, H., Xu, L., et al. (2014) Hydrothermal Synthesis of Oxidized G-c3N4 and Its Regulation of Photocata-
lytic Activity. Journal of Materials Chemistry A, 2, 19145-19149.
[36]Cao, L., Wang, R. and Wang, D. (2015) Synthesis and Characterization of Sulfur Self-Doped G-C3N4 with Efficient
Visible-Light Photocatalytic Activity. Materials Letters, 149, 50-53. https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.matlet.2015.02.119
[37]Chen, J., Hong, Z., Chen, Y., et al. (2015) One-Step Synthesis of Sulfur-Doped and Nitrogen-Deficient G-C3n4 Photo-
catalyst for Enhanced Hydrogen Evolution under Visible Light. Materials Letters, 145, 129-132.
[38]Hong, J., Xia, X., Wang, Y., et al. (2012) Mesoporous Carbon Nitride with In Situ Sulfur Doping for Enhanced Photo-
catalytic Hydrogen Evolution from Water under Visible Light. Journal of Materials Chemistry, 22, 15006-15012.
https://https://www.360docs.net/doc/1b4275077.html,/10.1039/c2jm32053c
[39]Liu, G., Niu, P., Sun, C.H., et al. (2010) Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped
Graphitic C3N4. Journal of the American Chemical Society, 132, 11642-11648. https://https://www.360docs.net/doc/1b4275077.html,/10.1021/ja103798k
[40]Wang, K., Li, Q., Liu, B., et al. (2015) Sulfur-Doped G-C3n4with Enhanced Photocatalytic Co2-Reduction Perfor-
mance. Applied Catalysis B: Environmental, 176, 44-52.
[41]Zhang, J., Sun, J., Maeda, K., et al. (2011) Sulfur-Mediated Synthesis of Carbon Nitride, Band-Gap Engineering and
Improved Functions for Photocatalysis. Energy & Environmental Science, 4, 675-678.
https://https://www.360docs.net/doc/1b4275077.html,/10.1039/C0EE00418A
[42]Guo, S., Deng, Z., Li, M., et al. (2016) Phosphorus-Doped Carbon Nitride Tubes with a Layered Micro-Nanostructure
for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Angewandte Chemie International Edition in English,
55, 1830-1834.
[43]Hu, S., Ma, L., You, J., et al. (2014) A Simple and Efficient Method to Prepare a Phosphorus Modified G-C3N4 Visible
Light Photocatalyst. RSC Advances, 4, 21657-21663.
[44]Lan, D.H., Wang, H.T., Chen, L., et al. (2016) Phosphorous-Modified Bulk Graphitic Carbon Nitride, Facile Prepara-
tion and Application as an Acid-Base Bifunctional and Efficient Catalyst for CO2 Cycloaddition with Epoxides. Car-
bon, 100, 81-89.
[45]Ma, T.Y., Ran, J., Dai, S., et al. (2015) Phosphorus-Doped Graphitic Carbon Nitrides Grown In Situ on Carbon-Fiber
Paper, Flexible and Reversible Oxygen Electrodes. Angewandte Chemie International Edition in English, 54,
4646-4650. https://https://www.360docs.net/doc/1b4275077.html,/10.1002/anie.201411125
[46]Ran, J.R., Ma, T.Y., Gao, G.P., et al. (2015) Porous P-Doped Graphitic Carbon Nitride Nanosheets for Synergistically
Enhanced Visible-Light Photocatalytic H2 Production. Energy & Environmental Science, 8, 3708-3717.
[47]Zhang, L.G., Chen, X.F., Guan, J., et al. (2013) Facile Synthesis of Phosphorus Doped Graphitic Carbon Nitride Po-
lymers with Enhanced Visible-Light Photocatalytic Activity. Materials Research Bulletin, 48, 3485-3491.
https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.materresbull.2013.05.040
[48]Zhang, Y. and Antonietti, M. (2010) Photocurrent Generation by Polymeric Carbon Nitride Solids, an Initial Step to-
wards a Novel Photovoltaic System. Asian Journal of Chemistry, 5, 1307-1311.
[49]Zhou, Y., Zhang, L., Liu, J., et al. (2015) Brand New P-Doped G-C3N4, Enhanced Photocatalytic Activity for H2 Evo-
lution and Rhodamine B Degradation under Visible Light. Journal of Materials Chemistry, 3, 3862-3867.
https://https://www.360docs.net/doc/1b4275077.html,/10.1039/C4TA05292G
[50]Lin, Z.Z. and Wang, X.C. (2013) Nanostructure Engineering and Doping of Conjugated Carbon Nitride Semiconduc-
tors for Hydrogen Photosynthesis. Angewandte Chemie International Edition, 52, 1735-1738.
[51]Sagara, N., Kamimura, S., Tsubota, T., et al. (2016) Photoelectrochemical CO2 Reduction by a P-Type Boron-Doped
G-C3n4 Electrode under Visible Light. Applied Catalysis B: Environmental, 192, 193-198.
https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.apcatb.2016.03.055
[52]Wang, Y., Li, H., Yao, J., et al. (2011) Synthesis of Boron Doped Polymeric Carbon Nitride Solids and Their Use as
Metal-Free Catalysts for Aliphatic C-H Bond Oxidation. Chemical Science, 2, 446-450.
https://https://www.360docs.net/doc/1b4275077.html,/10.1039/C0SC00475H
[53]Yan, S.C., Li, Z.S. and Zou, Z.G. (2010) Photodegradation of Rhodamine B and Methyl Orange over Boron-Doped
G-C3N4 under Visible Light Irradiation. Langmuir, 26, 3894-3901.
[54]Lin, H., Deng, W., Zhou, T., et al. (2015) Iodine-Modified Nanocrystalline Titania for Photo-Catalytic Antibacterial
Application under Visible Light Illumination. Applied Catalysis B: Environmental, 176, 36-43.
[55]Dong, G., Zhao, K. and Zhang, L. (2012) Carbon Self-Doping Induced High Electronic Conductivity and Photoreactiv-
ity of G-C3N4. Chemical Communications, 48, 6178-6180. https://https://www.360docs.net/doc/1b4275077.html,/10.1039/c2cc32181e
游然等[56]Fang, S., Xia, Y., Lv, K., et al. (2016) Effect of Carbon-Dots Modification on the Structure and Photocatalytic Activity
of G-C3N4. Applied Catalysis B: Environmental, 185, 225-232.
[57]Fan, Y., Tan, X., Ou, X., et al. (2016) A Novel “On-Off” Electrochemiluminescence Sensor for the Detection of Con-
canavalin A Based on Ag-Doped G-C3N4. Electrochimica Acta, 202, 90-99.
https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.electacta.2016.04.013
[58]Deng, L. and Zhu, M. (2016) Metal Nitrogen (Co-G-C3N4) Doping of Surface-Modified Single-Walled Carbon Nano-
horns for Use as an Oxygen Reduction Electrocatalyst. RSC Advances, 6, 25670-25677.
https://https://www.360docs.net/doc/1b4275077.html,/10.1039/C5RA27895C
[59]Tian, J., Liu, Q., Asiri, A.M., et al. (2013) Ultrathin Graphitic Carbon Nitride Nanosheets, a Novel Peroxidase Mimetic,
Fe Doping-Mediated Catalytic Performance Enhancement and Application to Rapid, Highly Sensitive Optical Detec-tion of Glucose. Nanoscale, 5, 11604-11609.
[60]Tonda, S., Kumar, S., Kandula, S., et al. (2014) Fe-Doped and -Mediated Graphitic Carbon Nitride Nanosheets for
Enhanced Photocatalytic Performance under Natural Sunlight. Journal of Materials Chemistry A, 2, 6772.
https://https://www.360docs.net/doc/1b4275077.html,/10.1039/c3ta15358d
[61]Bhunia, M.K., Yamauchi, K. and Takanabe, K. (2014) Harvesting Solar Light with Crystalline Carbon Nitrides for Ef-
ficient Photocatalytic Hydrogen Evolution. Angewandte Chemie International Edition in English, 53,11001-11005.
https://https://www.360docs.net/doc/1b4275077.html,/10.1002/anie.201405161
[62]Ho, W., Zhang, Z., Lin, W., et al. (2015) Copolymerization with 2,4,6-Triaminopyrimidine for the Rolling-Up the
Layer Structure, Tunable Electronic Properties, and Photocatalysis of G-C3N4. ACS Applied Materials & Interfaces, 7, 5497-5505.
[63]Zhang, J., Zhang, M., Lin, S., et al. (2014) Molecular Doping of Carbon Nitride Photocatalysts with Tunable Bandgap
and Enhanced Activity. Journal of Catalysis, 310, 24-30. https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.jcat.2013.01.008
[64]Lu, X., Xu, K., Chen, P., et al. (2014) Facile One Step Method Realizing Scalable Production of G-C3N4 Nanosheets
and Study of Their Photocatalytic H2 Evolution Activity. Journal of Materials Chemistry A, 2, 18924-18928.
[65]She, X., Liu, L., Ji, H., et al. (2016) Template-Free Synthesis of 2D Porous Ultrathin Nonmetal-Doped G-C3n4 Nano-
sheets with Highly Efficient Photocatalytic H2 Evolution from Water under Visible Light. Applied Catalysis B: Envi-ronmental, 187, 144-153.
[66]Zhao, H., Yu, H., Quan, X., et al. (2014) Fabrication of Atomic Single Layer Graphitic-C3n4 and Its High Performance
of Photocatalytic Disinfection under Visible Light Irradiation. Applied Catalysis B: Environmental, 152, 46-50.
https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.apcatb.2014.01.023
[67]Cui, Y., Tang, Y. and Wang, X. (2015) Template-Free Synthesis of Graphitic Carbon Nitride Hollow Spheres for Pho-
tocatalytic Degradation of Organic Pollutants. Materials Letters, 161, 197-200.
[68]He, F., Chen, G., Zhou, Y., et al. (2015) The Facile Synthesis of Mesoporous G-C3n4 with Highly Enhanced Photoca-
talytic H2 Evolution Performance. Chemical Communications, 51, 16244-16246.
[69]Li, J., Cao, C. and Zhu, H. (2007) Synthesis and Characterization of Graphite-Like Carbon Nitride Nanobelts and Na-
notubes. Nanotechnology, 18, Article ID: 115605.
[70]Ma, T.Y, Dai, S., Jaroniec, M., et al. (2014) Graphitic Carbon Nitride Nanosheet-Carbon Nanotube Three-Dimensional
Porous Composites as High-Performance Oxygen Evolution Electrocatalysts. Angewandte Chemie International Edi-tion in English, 53, 7281-7285. https://https://www.360docs.net/doc/1b4275077.html,/10.1002/anie.201403946
[71]Tahir, M., Cao, C., Mahmood, N., et al. (2014) Multifunctional G-C3N4 Nanofibers, a Template-Free Fabrication and
Enhanced Optical, Electrochemical, and Photocatalyst Properties. ACS Applied Materials & Interfaces, 6, 1258-1256.
[72]Wang, J., Zhang, C., Shen, Y., et al. (2015) Environment-Friendly Preparation of Porous Graphite-Phase Polymeric
Carbon Nitride Using Calcium Carbonate as Templates, and Enhanced Photoelectrochemical Activity. Journal of Ma-terials Chemistry A, 3, 5126-5131. https://https://www.360docs.net/doc/1b4275077.html,/10.1039/C4TA06778A
[73]Wu, X., Liu, C., Li, X., et al. (2015) Effect of Morphology on the Photocatalytic Activity of G-C3N4 Photocatalysts
under Visible-Light Irradiation. Materials Science in Semiconductor Processing, 32, 76-81.
https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.mssp.2014.11.047
[74]Yang, J., Wu, X., Li, X., et al. (2011) Synthesis and Characterization of Nitrogen-Rich Carbon Nitride Nanobelts by
Pyrolysis of Melamine. Applied Physics A, 105, 161-166.
[75]Sun, Z., Li, C., Yao, G., et al. (2016) In Situ Generated G-C3n4/Tio2 Hybrid over Diatomite Supports for Enhanced
Photodegradation of Dye Pollutants. Material Design, 94, 403-409.
[76]Wang, W., Yu, J.C., Xia, D., et al. (2013) Graphene and G-C3n4 Nanosheets Cowrapped Elemental Alpha-Sulfur as a
Novel Metal-Free Heterojunction Photocatalyst for Bacterial Inactivation under Visible-Light. Environmental Science & Technology, 47, 8724-8732. https://https://www.360docs.net/doc/1b4275077.html,/10.1021/es4013504
游然等
[77]Zhang, J., Zhang, M., Sun, R.Q., et al. (2012) A Facile Band Alignment of Polymeric Carbon Nitride Semiconductors
to Construct Isotype Heterojunctions. Angewandte Chemie International Edition in English, 51, 10145-10149.
[78]Zhang, S, Li, J., Wang, X., et al. (2014) In Situ Ion Exchange Synthesis of Strongly Coupled Ag@AgCl/g-C3N4 Porous
Nanosheets as Plasmonic Photocatalyst for Highly Efficient Visible-Light Photocatalysis. ACS Applied Materials &
Interfaces, 6, 22116-22125.
[79]Zhang, Y., Wen, R., Guo, D., et al. (2016) One-Step Facile Fabrication and Photocatalytic Activities of ZnS@g-C3N4
Nanocomposites from Sulfatotris (Thiourea) Zinc(II) Complex. Applied Organometallic Chemistry, 30, 160-166.
[80]Li, Z., Wang, J., Zhu, K., et al. (2015) Ag/G-C3N4 Composite Nanosheets, Synthesis and Enhanced Visible Photocata-
lytic Activities. Materials Letters, 145, 167-170. https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.matlet.2015.01.058
[81]Liu, L., Qi, Y., Yang, J., et al. (2015) An AgI@g-C3N4 Hybrid Core@Shell Structure,Stable and Enhanced Photocata-
lytic Degradation. Applied Surface Science, 358, 319-327.
[82]Liu, S., Sun, H., O'donnell, K., et al. (2016) Metal-Free Melem/G-C3n4 Hybrid Photocatalysts for Water Treatment.
Journal of Colloid and Interface Science, 464, 10-17. https://https://www.360docs.net/doc/1b4275077.html,/10.1016/j.jcis.2015.11.003
[83]Liu, X., Jian, X., Yang, H., et al. (2016) A Novel Method for Evaluating the Photoelectrocatalytic Performance of Re-
duced Graphene Oxide/Protonated G-C3N4 Composites. Materials Letters, 176,209-212.
[84]Ma, H., Li, Y., Li, S., et al. (2015) Novel PO Codoped G-C3n4 with Large Specific Surface Area, Hydrothermal Syn-
thesis Assisted by Dissolution-Precipitation Process and Their Visible Light Activity under Anoxic Conditions. Ap-
plied Surface Science, 357, 131-138.
[85]Huang, Z., Li, F., Chen, B., et al. (2015) Porous and Low-Defected Graphitic Carbon Nitride Nanotubes for Efficient
Hydrogen Evolution under Visible Light Irradiation. RSC Advances, 5, 102700-102706.
[86]Hu, S., Ma, L., Xie, Y., et al. (2015) Hydrothermal Synthesis of Oxygen Functionalized S-P Codoped G-C3N4 Nano-
rods with Outstanding Visible Light Activity under Anoxic Conditions. Dalton Transactions, 44, 20889-20897.
https://https://www.360docs.net/doc/1b4275077.html,/10.1039/C5DT04035C
[87]Ye, S., Wang, R., Wu, M.Z., et al. (2015) A Review on G-C3N4 for Photocatalytic Water Splitting and CO2 Reduction.
Applied Surface Science, 358, 15-27.
[88]Jun, Y.S., Park, J., Lee, S.U., et al. (2013) Three-Dimensional Macroscopic Assemblies of Low-Dimensional Carbon
Nitrides for Enhanced Hydrogen Evolution. Angewandte Chemie International Edition in English, 52, 11083-11087.
[89]Jun, Y.S., Lee, E.Z., Wang, X., et al. (2013) From Melamine-Cyanuric Acid Supramolecular Aggregates to Carbon
Nitride Hollow Spheres. Advanced Functional Materials, 23, 3661-3667.
[90]Shen, J.S., Cai, Q.G., Jiang, Y.B., et al. (2010) Anion-Triggered Melamine Based Self-Assembly and hydrogel. Chem-
ical Communications, 46, 6786-6788. https://https://www.360docs.net/doc/1b4275077.html,/10.1039/c0cc02030c
[91]You, R., Dou, H.L., Chen, L., Zheng, S.H. and Zhang, Y.P. (2017) Graphitic Carbon Nitride with S and O Codoping
for Enhanced Visible Light Photocatalytic Performance. RSC Advances, 7, 15842-15850.
期刊投稿者将享受如下服务:
1. 投稿前咨询服务(QQ、微信、邮箱皆可)
2. 为您匹配最合适的期刊
3. 24小时以内解答您的所有疑问
4. 友好的在线投稿界面
5. 专业的同行评审
6. 知网检索
7. 全网络覆盖式推广您的研究
投稿请点击:https://www.360docs.net/doc/1b4275077.html,/Submission.aspx
期刊邮箱:japc@https://www.360docs.net/doc/1b4275077.html,
石墨相氮化碳的制备及其光催化性能的研究
学校代码:10255 学号:2131347 DONGHUA UNIVERSITY 硕士学位论文 石墨相氮化碳的制备及其光催化性能的研究Preparation and Photocatalytic Properties of Graphite phase Carbon Nitride 专业:环境工程 作者:史振涛 导师:许士洪(副教授) 完成日期:2015年5月
东华大学 硕士学位论文答辩委员会成员名单
东华大学学位论文原创性声明 本人郑重声明:我恪守学术道德,崇尚严谨学风。所呈交的学位论文,是本人在导师的指导下,独立进行研究工作所取得的成果。除文中已明确注明和引用的内容外,本论文不包含任何其他个人或集体已经发表或撰写过的作品及成果的内容。论文为本人亲自撰写,我对所写的内容负责,并完全意识到本声明的法律结果由本人承担。 学位论文作者签名: 日期:年月日
东华大学学位论文版权使用授权书学位论文作者完全了解学校有关保留、使用学位论文的规定,同意学校保留并向国家有关部门或机构送交论文的复印件和电子版,允许论文被查阅或借阅。本人授权东华大学可以将本学位论文的全部或部分内容编入有关数据库进行检索,可以采用影印、缩印或扫描等复制手段保存和汇编本学位论文。 保密□,在年解密后适用本版权书。 本学位论文属于 不保密□。 学位论文作者签名:指导教师签名: 日期:年月日日期:年月
石墨相氮化碳的制备及其光催化性能的研究 摘要 近年来,半导体光催化技术得到了快速的发展。聚合物半导体石墨相氮化碳(g-C3N4)因其无毒、催化活性高、氧化能力强、且具有优异的化学稳定性和独特的电子能带结构、不含金属组分等优点而得到广泛研究。但是由于聚合物的材料特性,将g-C3N4作为光催化剂还存在如比表面积小、光生电子-空穴复合严重、量子效率低和禁带宽度较大而不能有效利用太阳光等严重制约其在能源、环境光催化领域的大规模推广应用的问题。因此,为了更好的利用太阳光,对g-C3N4进行制备优化及改性以得到较高可见光响应的光催化剂是非常必要的。 在本论文中,作者通过2种简单温和的方法制备出了在可见光下有较好光催化活性的石墨相氮化碳(g-C3N4)光催化剂。同时,结合XRD、UV-Vis、SEM、TEM、BET、TGA等实验手段,研究了不同制备条件对g-C3N4光催化剂的结构及催化性能的影响。结果如下: 1、以双氰胺为原料,通过改变煅烧时间及煅烧温度制备g-C3N4光催化剂。XRD结果表明在一定温度区间内随着煅烧温度的升高g-C3N4的结晶度先变好后变差。UV-Vis的结果表明制备的g-C3N4光催化剂吸收边在460 nm左右。实验还研究了不同煅烧温度、时间对g-C3N4光催化性能的影响,并初步探究了其催化机理。可见光下降解
原子掺杂改善石墨相氮化碳光催化性能的 研究进展
Journal of Advances in Physical Chemistry 物理化学进展, 2017, 6(2), 84-96 Published Online May 2017 in Hans. https://www.360docs.net/doc/1b4275077.html,/journal/japc https://https://www.360docs.net/doc/1b4275077.html,/10.12677/japc.2017.62011 Research Progress on Improving the Photocatalysis of Graphite-C3N4 via O, S and P Doping Ran You, Lu Chen, Yongping Zhang Faculty of Materials and Energy, Southwest University, Chongqing Received: May 4th, 2017; accepted: May 21st, 2017; published: May 24th, 2017 Abstract Graphitic arbon nitride (g-C3N4) has attracted extensive attention in the field of photocatalysis because of its unique atomic and electronic structures. Some intrinsic characteristics, such as small specific surface area and rapid recombination of the photogenerated electron-hole pair, re-strict its application. It is a valid pathway to improve the photocatalytic performance of g-C3N4 by changing its physical and chemical properties via atomic doping. In this paper, we overview the ways to optimize the photocatalysis of g-C3N4, and focus on the techniques and effect of atomic doping. Finally, the research perspective on the g-C3N4 is discussed. Keywords Template, Graphitic-C3N4, Atomic Doping, Photocatalytic Performance 原子掺杂改善石墨相氮化碳光催化性能的 研究进展 游然,陈露,张永平 西南大学材料与能源学部,重庆 收稿日期:2017年5月4日;录用日期:2017年5月21日;发布日期:2017年5月24日 摘要 石墨相氮化碳(g-C3N4)独特的结构使其在光催化性能上具有一定的优越性,使其研究受到了广泛的关注。 文章引用: 游然, 陈露, 张永平. 原子掺杂改善石墨相氮化碳光催化性能的研究进展[J]. 物理化学进展,2017, 6(2):
石墨相氮化碳的改性及光催化降解有机污染物的研究
石墨相氮化碳的改性及光催化降解有机污染物的研究 光催化技术可用于分解水产氢和降解有机污染物,是解决能源危机和环境污染问题的新型绿色技术。半导体光催化材料石墨相氮化碳 (g-C3N4)是一种非金属碳氮聚合物,因其具有合适的禁带宽度、良好的化学和热稳定性、制备方法简单等特点。 然而,氮化碳材料具有对可见光吸收能力欠佳,光生电子与空穴重组效率较高等缺陷,严重制约了它的实际应用。因此,本文通过简单快捷的方法对 g-C3N4从尺寸调控、构建异质结和负载助催化剂等方面改性,显著提高其光催化降解有机污染物的性能,具有重要的现实意义。 本文主要研究内容及结论如下:以三聚氰胺为前驱材料制备了体相 g-C3N4,通过煅烧、超声的方法对体相 g-C3N4进行剥离,得到尺寸较小、片层较少的 g-C3N4纳米片;优化了煅烧次数和超声时间,获得了最佳的制备工艺条件,改善了g-C3N4催化降解罗丹明B (Rhodamine B,Rh B)的性能,探讨了其在可见光下降解Rh B的机制。结果表明,二次煅烧并超声处理的方法有效提高了g-C3N4材料降解Rh B的活性。 通过高温煅烧双氰胺得到纯相g-C3N4,通过简单的原位沉淀法,将Ag2WO4成功附着在 g-C3N4片层表面,得到 Ag2WO4/g-C3N4异质结光催化剂,制备过程中未改变g-C3N4的整体形貌和晶体结构,
g-C3N4基光催化剂的制备及性能的研究
目录 摘要................................................................................................................................. I Abstract ............................................................................................................................. II 目录.................................................................................................................................. III 第一章绪论. (1) 1.1 引言 (1) 1.2 光催化材料综述 (1) 1.2.1 光催化机理 (2) 1.2.2 光催化材料在能源环境领域的应用 (3) 1.3 g-C3N4概述 (4) 1.3.1 g-C3N4的研究背景 (4) 1.3.2 g-C3N4的结构和性质 (4) 1.3.3 g-C3N4的制备 (5) 1.3.4 g-C3N4的研究现状 (5) 1.4 MoS2概述 (7) 1.4.1 MoS2的研究背景 (7) 1.4.2 MoS2的结构和性质 (8) 1.4.3 MoS2的制备 (8) 1.4.4 MoS2的研究现状 (9) 1.5 本文选题意义及内容 (11) 第二章亮橙色管状氮化碳光催化剂的制备及性能的研究 (12) 2.1 引言 (12) 2.2 实验试剂及设备 (12) 2.2.1 实验试剂 (12) 2.2.2 实验设备 (12) 2.3 材料的合成 (13) 2.3.1 g-C3N4的合成 (13) 2.3.2 管状氮化碳的合成 (13) 2.4 光催化剂活性的评价 (13) 2.5 结果与讨论 (14) 2.6 本章小结 (22) 第三章具备高效电荷分离能力的MoS2/g-C3N4复合催化剂的制备及性能的研究23 3.1 引言 (23) 3.2 实验试剂及设备 (23) 3.2.1 实验试剂 (23)
石墨相氮化碳光催化剂改性的研究进展
第32卷第2期2019年4月 Vol.32No.2 Apr.2019 投稿网址:https://www.360docs.net/doc/1b4275077.html, 石油化工高等学校学报 JOURNAL OF PETROCHEMICAL UNIVERSITIES 石墨相氮化碳光催化剂改性的研究进展 李昱慧,张静,张昱屾,陈常东 (辽宁石油化工大学化学化工与环境学部,辽宁抚顺113001) 摘要:石墨相氮化碳(g?C3N4)是具有二维层状结构的材料,作为非金属半导体光催化剂有可见光吸收、能带等级可调变、化学稳定性高、绿色环保无污染等诸多优点。结合近几年国内外g?C3N4研究人员取得的最新成果及研究进展,从掺杂物质结构的角度综述了分子结构改变、单质掺杂、半导体负载及三元复合等g?C3N4的多种改性方法及催化机理,并对未来如何提升g?C3N4的光催化性能进行展望。 关键词:催化剂;石墨相氮化碳;复合材料;降解 中图分类号:TB333文献标志码:A doi:10.3969/j.issn.1006?396X.2019.02.001 The Research Progress of Modified g?C3N4Composite Photocatalyst Li Yuhui,Zhang Jing,Zhang Yushen,Chen Changdong (College of Chemistry Chemical Industry&Environment,Liaoning Shihua University,Fushun Liaoning113001,China)Abstract:Graphitic carbon nitride(g?C3N4)possesses a unique two?dimensional structure,which is used as a kind of excellent non?metallic semiconductor photocatalysts,owing to its visible light absorption,adjustable energy band grade,high stability,and so on.This review focus on recent research results and progress,and modification and catalytic mechanism related to photocatalysts are summarized based on microstructure changing,elemental doping,semiconductor loading,and multiphase composite of g?C3N4.Finally,the review presents the viewpoint for the development of g?C3N4?based photocatalytic performance. Keywords:Catalyzer;g?C3N4;Compound material;Degradation 随着经济社会快速发展,环境污染越来越严重,减少环境污染建设绿色中国一直是科研工作者的首要课题。自1972年以来,研究人员发现TiO2具有可见光吸收性能,并将其用作光催化降解污染物,但由于TiO2具有禁带宽度较大、太阳能利用率低、光生电子空穴复合率高等缺点,极大限制了它的实际应用。因此科学家们开始寻找比TiO2光催化活性更加优异的半导体光催化剂。 石墨相氮化碳作为本世纪备受科学家们青睐的非金属半导体光催化剂具有诸多优点:较宽的可见光吸收、带间能级可调变、化学稳定性高、边缘丰富N源、无毒无害等。g?C3N4作为一种环境友好型碳材料常被研究者应用于降解有机污染物及分解水产氢[1]。在可见光催化条件下光生电子空穴与氧生成超氧自由基和羟基自由基等活性物种,能够快速降解有机污染物且无二次污染。但单体g?C3N4存在禁带宽度大、光生载流子与空穴复合率高、可见光利用率低等缺点。因此,想获得性能更加优异的复合光催化剂就需要对g?C3N4进行高效改性。本文简要综述了g?C3N4的制备方法,从掺杂物由低到高的复杂程度,即分子结构改变、单质或非金属半导体负载及三元复合等多种角度,对g?C3N4改性后光催化剂的光催化活性和催化机理进行分析,最后根据g?C3N4的研究现状对其未来的研究方向进行展望。 文章编号:1006?396X(2019)02?0001?07 收稿日期:2018?10?18修回日期:2018?11?07 基金项目:国家自然科学基金(21573101);辽宁省高端人才项目([2015]153);辽宁省高等学校创新人才支持计划项目(LR2017011);辽宁省百千万人才工程项目([2017]96)。 作者简介:李昱慧(1992?),女,硕士研究生,从事复合材料的构筑及光催化性能分析研究;E?mail:827444605@https://www.360docs.net/doc/1b4275077.html,。 通信联系人:张静(1980?),女,博士,教授,博士生导师,从事光催化、生物质催化及先进清洁能源材料开发与利用研究;E?mail:jingzhang_dicp@https://www.360docs.net/doc/1b4275077.html,。
以石墨相氮化碳为基础的聚合物光催化剂(4)
以石墨相氮化碳为基础的聚合物光催化剂 摘要 半导体光催化是一种很吸引大家研究兴趣的方法来解决全世界范围内的能源紧缺和环境污染问题。自从石墨相C3N4在2009年被用于可见光光催化分解水以来,C3N4光催化已经成为一个非常热门的研究话题。这篇综述总结了C3N4设计制备方面的一些研究进展,也对其在能源和环境方面的应用做了一个全面的概述。 Polymeric Photocatalysts Based on Graphitic Carbon Nitride Semiconductor-based photocatalysis is considered to be an attractive way for solving the worldwide energy shortage and environmental pollution issues. Since the pioneering work in 2009 on graphitic carbon nitride (g-C 3 N 4 ) for visible-light photocatalytic water splitting, g-C 3 N 4 -based photocatalysis has become a very hot research topic. This review summarizes the recent progress regarding the design and preparation of g-C 3 N 4 -based photocatalysts, including the fabrication and nanostructure design of pristine g-C 3 N 4 , bandgap engineering through atomic-level doping and molecular-level modifi cation, and the preparation of g-C 3 N 4 -based semiconductor composites. Also, the photocatalytic applications of g-C 3 N 4 -based photocatalysts in the fi elds of water splitting, CO 2 reduction, pollutant degradation, organic syntheses, and bacterial disinfection are reviewed, with emphasis on photocatalysis promoted by carbon materials, non-noble-metal cocatalysts, and Z-scheme heterojunctions. Finally, the concluding remarks are presented and some perspectives regarding the future development of g-C 3 N 4 -based photocatalysts are highlighted.
石墨相氮化碳光催化产氢研究进展
Chenmical Intermediate 当代化工研究 122 科研开发 2017·03 [2]R. Howarth,D.Anderson, J.Cloern, et al.2000. Nutrient pollution of coastalrivers, bays, and seas [J]. Issues in Ecology, 7: 1–15. [3]B. Kronvangl, et al.1996. Diffuse nutrient losses in denmark [J]. Water Sci.Technol.(G.B.), 33: 81–86. [4]杨林章,冯彦房,施卫明,薛利红,王慎强,宋祥甫,常志州.2013. 我国农业面源污染治理技术研究[J].中国生态农业学报, 2(1):96-101. [5]尹澄清,毛战坡.2002.用生态工程技术控制农村非点源水污染[J].应用生态学报,13(2):229-232. [6]贺缠生,傅伯杰,陈利顶.1998.非点源污染的管理与控制[J].环境科学,19(5): 87–96. [7]V. Simeonov, H. Puxbaum, S. Tsakovski, C. Sarbu, M. Kalina, 1999. Classification and receptor modeling of wet precipitation data from central Austrial (1984-1993)[J]. Environmetrics, 10: 137-152. [8]B.D. Eyre, P. Pepperell, 1999. A spatial intensive approach to water quality mornitoring in the Rous River catchment, NSW, Australia[J]. Journal of Environmental Management, 56(2):97-118. [9]R.B. Grayson, C.J. Gippel, B.L. Finlayson, et al. 1997. Catchment-wideimpacts on water quality: the use of ‘snapshot’ sampling during stable flow[J]. Journal of Hydrology, 199: 121–134. [10]D.E. Walling, and B.W. Webb, 1975. Spatial variation in water quality: a survey of the River[J]. Transaction of the Institute of British Geographers, 65:155-171. [11]V. Simeonov, J.A. Stratis, C. Samara, G. Zachariadis, 2003. Assessment of the surface water quality in North Greece[J]. Water Research, 37(17):4119-4124. [12]R. Reghunath, T.R.S. Murthy, The utility of multivariate statistical techniques in hydrogeochemical studies: an example from Karnataka India[J]. Water Research, 36:2437-2442. [13]D.A. Wudderlin, P.D. Mariaodel, V.A. Maria, M.D.A. Bistoni, 2001. Pattern recognition techniques for the evaluation of spatial and temporal variation in water quality: a case study Suqui a River Basin(Cordoba-Argentina)[J]. Water Research, 35(2):2881-2894. [14]J.L. Taraba, J.S. Dinger, L.V.A. Sendlein, G.K. Felton, 1996. Land use impacts on water quality from small agricultural watersheld in Kentuchy. ASAE paper. [15]F. William, and Hansen. 2001. Identifying stream types and management implications[J]. Forest Ecology and Management, 143(1):39-46. ?【作者简介】 刘玉超(1976~),男,上海太和水环境科技发展有限公司;研究方向:富营养化水体生态修复。 (责任编辑(牛玉娟) 石墨相氮化碳光催化产氢研究进展 *赵焕新* 代佳莉 钞泽睿 朱阳晨 (沈阳化工大学 辽宁 110142) 摘要:光催化产氢是未来解决能源危机的有效方法。本文介绍了光催化产氢的基本原理,影响效率的因素及石墨相氮化碳催化的研究进 展。 关键词:光催化;产氢;石墨相氮化碳中图分类号:T 文献标识码:A Development of Graphite Phase Carbon Nitride Photocatalysis Hydrogen Generation Study Zhao Huanxin, Dai Jiali, Chao Zerui, Zhu Yangchen, (Shenyang University of Chemical Engineering, Liaoning, 110142) Abstract :Photocatalysis hydrogen generation is the effective method for solving the energy crisis in future. In this paper, it has introduced the basic theory of photocatalysis hydrogen generation, the factors influencing efficiency and the research development of graphite phase carbon nitride catalysis. Key words :photocatalysis ;hydrogen generation ;graphite phase carbon nitride 1.引言 当今社会,能源对社会经济的发展及人类生活水平均起到至关重要的作用。传统的一次能源,以煤、石油、天然气为代表的化石燃料日益短缺,面临枯竭。开发新型的可替代能源是实现社会可持续发展的必经之路。相比于核能、风能、水能等二次能源,氢能具有热值高(142kJ/kg)、稳定性高、使用过程清洁,无二次污染等优势。因此,针对产氢、储氢等领域的研究日益成为热点。目前已经出现的产氢技术 主要包括电化学产氢、厌氧微生物发酵产氢及光催化产氢等。相比于前两种技术,光催化产氢能够直接以水为生产原料,在催化剂作用下将光能转化为氢能。而在自然界中,太阳光被认为是一种取之不尽用之不竭的光源。因此,开展光催化产氢技术的研究具有重要的科学意义及战略意义。 2.光催化产氢的基本原理 光催化产氢技术最早由日本科学家Fujishima利用TiO 2 半导体催化剂,在紫外光与电场的联合作用下,实现了分解 上接第121页 下转第123页
石墨相氮化碳材料及其光催化应用_苗阳森
0引言 化石能源危机是当前我国实现可持续发展面临的严重问题,寻找解决问题的有效途径具有重要意义。近年来,关于太阳能利用的研究,特别是太阳能光催化研究的发展十分活跃,尤其是在半导体光催化剂研究方面。目前,光催化领域使用的催化剂多为金属半导体和过渡金属复合物,存在太阳光利用率低、活性低和稳定性差等缺点。而氮化碳具有硬度高、密度低、氮含量高、化学稳定性好以及耐摩擦等优点,可作为高性能的耐摩擦材料,合成金属氮化物的氮源[1-5];同时,由于具有独特的光学和电子性质,在材料、光学、电子等领域中具有诱人的应用前景,如储能材料、传感器、金属防腐等[6-9];氮化碳作为有机半导体非金属光催化剂在光催化分解水和降解有机污染物等领域具有简单易行、符合环保要求以及成本低的优点,在解决能源开发和环境治理问题上具有重要意义。 与传统的无机半导体光催化剂比较,氮化碳具有化学性质稳定、热稳定性强、可见光利用率高、制备简易和原料丰富且无毒等特点。其将太阳能转化为化学能等其它形式能量的光催化性能和光催化降解有机污染物的作用为当下解决煤、石油等能源危机以及环境污染等人类亟待解决的问题提供了有效途径。 1氮化碳的研究历史 氮化碳是文献中报道的最古老的聚合物之一。关于它的研究最早可以追溯到1834年,Liebig 把一种由Berzelius合成出的聚合衍生物命名为“melon”[10]。1922年,Franklin通过热解硫氰酸汞制备了一种无定形的C3N4化合物,并提出这种化合物可能具有类似石墨的结构[11]。此后,研究者希望通过硫氰酸盐、三嗪类和七嗪类化合物的热解制备出氮化碳,但是都没能得到明确的晶体结构。1985年,M.L.Cohen根据半经验公式估算出C3N4四面体化合物的体弹性模量值为461~483GPa[12]。 1989年,Liu等[13]以β-Si3N4为结构模型,用C 代替Si,在局域态密度近似下采用第一性赝势能带法,从理论上预言β-C3N4的硬度与金刚石相当之后,氮化碳的研究进入新的时期。1993年,Fox 等[14]成功在实验室合成氮化碳薄膜,并证实其硬度超过金刚石成为世界上最硬的新材料。1996年,Teter等[15]通过第一性原理计算认为,氮化碳晶体可能具有五种结构:α相、β相、立方相、准立 新材料 石墨相氮化碳材料及其光催化应用 苗阳森,卢春山*,李小年 (浙江工业大学工业催化研究所,绿色化学合成技术国家重点实验室培育基地,浙江杭州310032) 摘要:石墨相氮化碳具有独特的电子能带结构和优异的化学稳定性,作为一种不含金属成分的新型可见光光催化剂,在光催化领域有着广泛的应用前景。介绍了近年来石墨相氮化碳的研究现状,重点探讨其合成方法、结构特性和其相关的衍生物以及在光催化中的应用。 关键词:石墨相氮化碳;衍生物;光催化 文章编号:1006-4184(2016)2-0039-07 收稿日期:2015-05-18 作者简介:苗阳森(1992-),男,河南驻马店人,硕士生。E-mail:miaowangmiaowang@https://www.360docs.net/doc/1b4275077.html,。
