(完整版)化学专业英语
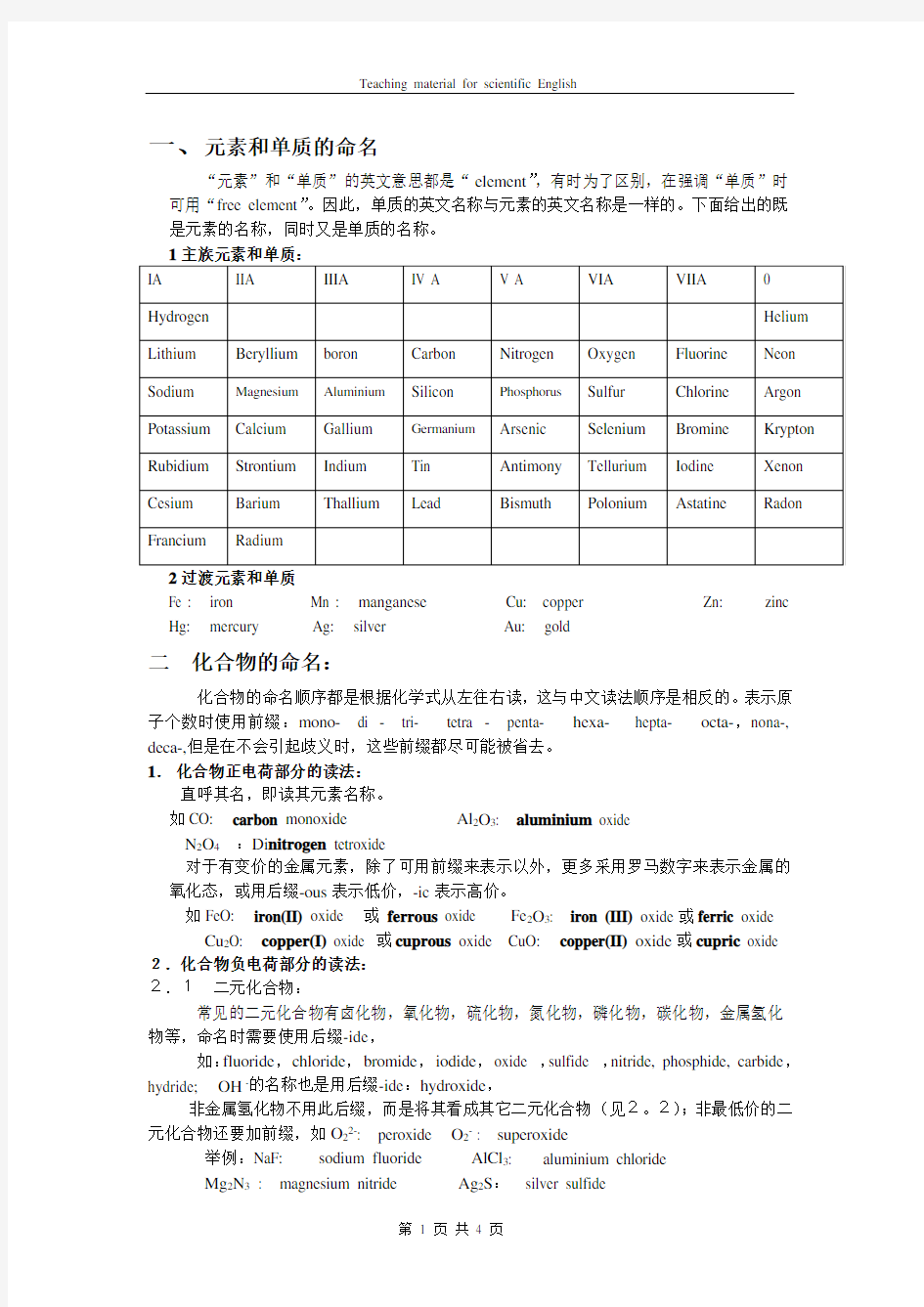

一、元素和单质的命名
“元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质”时可用“free element”。因此,单质的英文名称与元素的英文名称是一样的。下面给出的既是元素的名称,同时又是单质的名称。
2过渡元素和单质
Fe : iron Mn : manganese Cu: copper Zn: zinc Hg: mercury Ag: silver Au: gold
二化合物的命名:
化合物的命名顺序都是根据化学式从左往右读,这与中文读法顺序是相反的。表示原子个数时使用前缀:mono-di -tri- tetra -penta- hexa-hepta- octa-,nona-, deca-,但是在不会引起歧义时,这些前缀都尽可能被省去。
1.化合物正电荷部分的读法:
直呼其名,即读其元素名称。
如CO: carbon monoxide Al2O3: aluminium oxide
N2O4:Di nitrogen tetroxide
对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous表示低价,-ic表示高价。
如FeO: iron(II) oxide 或ferrous oxide Fe2O3: iron (III) oxide或ferric oxide Cu2O: copper(I) oxide 或cuprous oxide CuO: copper(II) oxide或cupric oxide 2.化合物负电荷部分的读法:
2.1二元化合物:
常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化物,金属氢化物等,命名时需要使用后缀-ide,
如:fluoride,chloride,bromide,iodide,oxide ,sulfide ,nitride, phosphide, carbide,hydride; OH -的名称也是用后缀-ide:hydroxide,
非金属氢化物不用此后缀,而是将其看成其它二元化合物(见2。2);非最低价的二元化合物还要加前缀,如O22-: peroxide O2- : superoxide
举例:NaF: sodium fluoride AlCl3: aluminium chloride
Mg2N3: magnesium nitride Ag2S:silver sulfide
CaC2: calcium carbide Fe(OH)2:iron(II) hydroxide
有些物质常用俗称,如NO nitric oxide N2O nitrous oxide
2.2非金属氢化物
除了水和氨气使用俗称water,ammonia以外,其它的非金属氢化物都用系统名称,命名规则根据化学式的写法不同而有所不同。对于卤族和氧族氢化物,H在化学式中写在前面,因此将其看成另一元素的二元化合物。
举例:HF hydrogen fluoride HCl hydrogen chloride
HBr hydrogen bromide HI hydrogen iodide
H2S hydrogen sulfide H2Se hydrogen selenide
H2Te hydrogen telluride
对于其它族的非金属氢化物,H在化学式中写在后面,可加后缀—ane,氮族还可加-ine 举例:PH3: phosphine或phosphane AsH3: arsine或arsane
SbH3: stibine或stibane BiH3: bismuthane
CH4: methane SiH4: silane B2H6: diborane
2.3无氧酸
命名规则:hydro-词根-ic acid
举例:HCl: hydrochloric acid
H2S : hydrosulfuric acid
2.4含氧酸与含氧酸根阴离子
化学专业英语用前后缀的不同组合显示不同价态的含氧酸和含氧酸根阴离子,价态相同的含氧酸及含氧酸根阴离子具有相同的前缀,不同的后缀。
高某酸per-ic 正酸–ic亚酸-ous 次酸hypo-ous
高某酸根per-ate 正酸根–ate 亚酸根-ite 次酸根hypo-ite
其它的前缀还有ortho-正meta- 偏thio-硫代
举例:HClO4 perchloric acid ClO4- perchlorate ion
HClO3 chloric acid ClO3- chlorate ion
HClO2 chlorous acid ClO2- chlorite ion
HClO hypochlorous acid ClO-hypochlorite ion
H2SO4 sulfuric acid H2SO3sulfurous acid
HNO3nitric acid HNO2nitrous acid
HPO3 metaphosphoric acid S2O32- thiosulfate ion
2.5盐
正盐:根据化学式从左往右分别读出阳离子和阴离子的名称。
如FeSO4 iron(II) sulfate KMnO4 potassium permanganate
酸式盐:同正盐的读法,酸根中的H读做hydrogen,氢原子的个数用前缀表示。
如NaHCO3: sodium hydrogen carbonate 或sodium bicarbonate
NaH2PO4:sodium dihydrogenphosphate
复盐:同正盐的读法,并且阳离子按英文名称的第一个字母顺序读。
如KNaCO3: potassium sodium carbonate
NaNH4HPO4: ammonium sodium hydrogen phosphate
水合盐:结晶水读做water或hydrate
如AlCl3.6H2O: aluminum chloride 6-water或aluminum chloride hexahydrate
AlK(SO4) 212H2O aluminium potassium sulphate 12-water
三物理性质(physical properties)
colour: colorless, red-brown, violet-black, purple-black, pale yellow, dark brown
smell: odorless, pungent, penetrating, offensive, choking, bitter, sour, sweet
state: solid, liquid, gas, gaseous, oily, crystalline, uncrystalline, molten, fused
solubility: soluble, insoluble, slightly soluble, very soluble ,
density: heavy, light , less dense, denser, greatly denser, slightly denser,
about the same dense
hardness: hard, soft , ductile, malleable
toxicity:toxic, poisonous
melting point, boiling point: high, low
conductiv ity: electrical conductivity ; thermal conductivity ; conductor ; insulator;
semiconductor
四化学性质(chemical properties)
stability: stable , unstable, reactive, unreactive
redox property: oxidizing ability, reducing ability, oxidizing agent(oxidant), reducing agent (reductant), oxidation, reduction, oxidation state, valence, strong, weak
acid-base property: acidic, basic, strong, weak, monohydroxy base, monoprotic acid,
五化学方程式(Chemical Equations)
1 反应名称:
Combination;decomposition;single displacement;double displacement;redox reaction;nonredox reaction;disproportionation;neutralization;exothermic reaction; endothermic reaction; reversible reaction; forward reaction; reverse reaction; spontaneous reaction; nonspontaneous reaction
2 反应条件:
heat; burn; ignite/ignition ; electrolyze/electrolysis; under/at ambient/room temperature; under standard pressure; with/in the presence of a catalyst
3 读法:
3.1 Nitrogen reacts with hydrogen to form ammonia at high temperature and pressure with the presence of a catalyst.
1 mol nitrogen reacts with 3 mol hydrogen to form
2 mol ammonia at high temperature and pressure with the presence of a catalyst.
3.2 Nitrogen combines with hydrogen to form ammonia at high temperature and pressure with the presence of a catalyst.
Ammonia decomposes to nitrogen and hydrogen at high temperature and pressure with the presence of a catalyst.
3.3 Reaction between nitrogen and hydrogen at high temperature and pressure with the presence of a catalyst gives ammonia.
At high temperature and pressure, reaction of nitrogen with hydrogen in the presence of a catalyst takes place.
六化学计算(Chemical Calculation)
1 化学术语:
atomic mass/weight ; molecular weight; amount (of substance); mole; number of moles; molar
mass; molar volume; concentration; molarity ; excess agent; limiting agent;reactant; product; yield;
2 数学术语:
+-×÷
运算名称addition subtraction mulplication division
动词读法add substract(ed)·from multiply(ied)·by divide(d)·by
介词读法plus minus times over
运算结果sum difference product quotient
0.001 o/zero point o o one
2/3 two thirds
=equals/is equal to
≈is approximately equal to
<less than
>greater than
x2x squared ; x3x cubed ; x-10x to the minus tenth power
100o c one hundred degrees centigrade
5% five percent (by mass, volume)
()round brackets/parentheses
[ ] square/angular brackets
{} braces
linear planar trigonal square tetrahedral
七化学实验(Chemical Experiments )
1 实验用品( equipments / apparatus )
烧瓶round-bottom/Florence flask 锥形瓶(conical) Erlenmeyer flask
三角漏斗funnel 长颈漏斗thistle tube 试管架test-tube rack
集气瓶bottle ; glass jar 滴定管burette 烧杯beaker
玻棒glass rod 洗瓶wash bottle 干燥管drying tube
试管刷test tube brush 温度计thermometer 火柴match
酒精灯burner 石棉网wire gauze 铁架台iron stand
指示剂indicator 酚酞phenolphthalein U型管U tube
石蕊litmus 甲基橙methyl orange 淀粉starch
橡皮塞rubber stopper 橡皮管rubber tube 滴管eye dropper
角匙spoon 蒸发皿evaporation dish 滤纸filter paper
研,棒mortar and pestle 量筒graduated cylinder 天平balance
2 实验报告:
aims ; principles/introduction ; procedures ; observations; conclusion/deduction
brisk effervescence, precipitate, milky, aqueous solution
3实验类型:
confirmative test ; inquiry test; qualitative analysis; quantitative analysis ; measurement / determination on
4实验操作:
collect gas (over water; upward displacement of air; downward delivery)
bubble gas through ; dry gas ; suck bac
(完整版)化学类专业英语词汇.doc
专业英语词汇 Unit 1TEXT A : Chemical Reactions and Group Reactions customary a. 通常的,惯例的 handle n.柄vt.触摸 handling n.处理,管理 derive vt.取得,得到,衍生 oxidate vt.使氧化oxidation n. satisfactory a.令人满意的,符合要求的 rapid a.快的,迅速的,动作快的 combustion n.燃烧 somewhat pron. ad. 一点点,几分,有点 effort n.努力 commercial a.商业的,商务的 undesirable a不.合需要的,不受欢迎的,讨厌的 retard vt.延迟,放慢,使停滞 transformer n.变压器 transform vt.改变,转变 automotive a.自动的,机动的,汽车的 cracked裂化的 sluge n.软泥,淤泥 stiff a.硬的,强烈的 extent 广度,程度 distillation n.蒸馏distill vt.vi. unrefined a.未精致,未提炼的 acidity n.酸味,酸性acidify vt. Vi. Involve vt. 包缠,卷缠 Fell=following Individual a.个人的,个体的 Presumable a可.假定的,可推测的 Destruction n.破坏,毁灭 Overall n。 a.全面的,综合的 Exceed 超过,胜过 Isolate vt.隔离,孤立,使离析iso—构词成分“均匀”“异构”“苯”Analyse vt. 分析,分解 Carbonyl 羰基 Carboxyl羧基 Hydroxyl羟基 Decomposition分解 Alkyl烷基,烃基
化学专业英语(修订版)翻译
01 THE ELEMENTS AND THE PERIODIC TABLE 01 元素和元素周期表 The number of protons in the nucleus of an atom is referred to as the atomic number, or proton number, Z. The number of electrons in an electrically neutral atom is also equal to the atomic number, Z. The total mass of an atom is determined very nearly by the total number of protons and neutrons in its nucleus. This total is called the mass number, A. The number of neutrons in an atom, the neutron number, is given by the quantity A-Z. 质子的数量在一个原子的核被称为原子序数,或质子数、周淑金、电子的数量在一个电中性原子也等于原子序数松山机场的总质量的原子做出很近的总数的质子和中子在它的核心。这个总数被称为大量胡逸舟、中子的数量在一个原子,中子数,给出了a - z的数量。 The term element refers to, a pure substance with atoms all of a single kind. T o the chemist the "kind" of atom is specified by its atomic number, since this is the property that determines its chemical behavior. At present all the atoms from Z = 1 to Z = 107 are known; there are 107 chemical elements. Each chemical element has been given a name and a distinctive symbol. For most elements the symbol is simply the abbreviated form of the English name consisting of one or two letters, for example: 这个术语是指元素,一个纯物质与原子组成一个单一的善良。在药房“客气”原子的原子数来确定它,因为它的性质是决定其化学行为。目前所有原子和Z = 1 a到Z = 107是知道的;有107种化学元素。每一种化学元素起了一个名字和独特的象征。对于大多数元素都仅仅是一个象征的英文名称缩写形式,一个或两个字母组成,例如: oxygen==O nitrogen == N neon==Ne magnesium == Mg
化学专业英语翻译1
01.THE ELEMENTS AND THE PERIODIC TABLE 01元素和元素周期 表。 The number of protons in the nucleus of an atom is referred to as the atomic number, or proton number, Z. The number of electrons in an electrically neutral atom is also equal to the atomic number, Z. The total mass of an atom is determined very nearly by the total number of protons and neutrons in its nucleus. This total is called the mass number, A. The number of neutrons in an atom, the neutron number, is given by the quantity A-Z. 原子核中的质子数的原子称为原子序数,或质子数,卓电子数的电中性的原子也等于原子序数Z,总质量的原子是非常接近的总数量的质子和中子在原子核。这被称为质量数,这个数的原子中的中子,中子数,给出了所有的数量 The term element refers to, a pure substance with atoms all of a single kind. To the chemist the "kind" of atom is specified by its atomic number, since this is the property that determines its chemical behavior. At present all the atoms from Z = 1 to Z = 107 are known; there are 107 chemical elements. Each chemical element has been given a name and a distinctive symbol. For most elements the symbol is simply the abbreviated form of
有机化学常用词缀及单词
有机化学专业英语词汇常用前后缀 acetal 醛缩醇 acetal- 乙酰 acid 酸 -al 醛 alcohol 醇 -aldehyde 醛 alkali- 碱 allyl 烯丙基 [propenyl(丙烯基)] alkoxy- 烷氧基 -amide 酰胺 amino- 氨基的 -amidine 脒 -amine 胺 -ane 烷 anhydride 酐 anilino- 苯胺基 aquo- 含水的 -ase 酶 -ate 含氧酸的盐、酯 -atriyne 三炔 azo- 偶氮 benzene 苯 bi- 在盐类前表示酸式盐 bis- 双 -borane 硼烷 bromo- 溴 butyl 丁基 -carbinol 甲醇 carbonyl 羰基 -carboxylic acid 羧酸 centi- 10-2 chloro- 氯代 cis- 顺式 condensed 缩合的、冷凝的 cyclo- 环 deca- 十 deci 10-1 -dine 啶 dodeca- 十二
-ene 烯 epi- 表 epoxy- 环氧 -ester 酯 -ether 醚 ethoxy- 乙氧基 ethyl 乙基 fluoro- 氟代 form 仿 -glycol 二醇 hemi- 半 hendeca- 十一 hepta- 七 heptadeca- 十七 hexa- 六 hexadeca- 十六 -hydrin 醇 hydro- 氢或水 hydroxyl 羟基 hypo- 低级的,次 hyper- 高级的,高 -ic 酸的,高价金属 -ide 无氧酸的盐,酰替胺,酐-il 偶酰 -imine 亚胺 iodine 碘 iodo- 碘代 iso- 异,等,同 -ite 亚酸盐 keto- 酮 ketone 酮 -lactone 内酯 mega- 106 meta- 间,偏 methoxy- 甲氧基 methyl 甲基
《化学工程与工艺专业英语》课文翻译 完整版
Unit 1 Chemical Industry 化学工业 1.Origins of the Chemical Industry Although the use of chemicals dates back to the ancient civilizations, the evolution of what we know as the modern chemical industry started much more recently. It may be considered to have begun during the Industrial Revolution, about 1800, and developed to provide chemicals roe use by other industries. Examples are alkali for soapmaking, bleaching powder for cotton, and silica and sodium carbonate for glassmaking. It will be noted that these are all inorganic chemicals. The organic chemicals industry started in the 1860s with the exploitation of William Henry Perkin‘s discovery if the first synthetic dyestuff—mauve. At the start of the twentieth century the emphasis on research on the applied aspects of chemistry in Germany had paid off handsomely, and by 1914 had resulted in the German chemical industry having 75% of the world market in chemicals. This was based on the discovery of new dyestuffs plus the development of both the contact process for sulphuric acid and the Haber process for ammonia. The later required a major technological breakthrough that of being able to carry out chemical reactions under conditions of very high pressure for the first time. The experience gained with this was to stand Germany in good stead, particularly with the rapidly increased demand for nitrogen-based compounds (ammonium salts for fertilizers and nitric acid for explosives manufacture) with the outbreak of world warⅠin 1914. This initiated profound changes which continued during the inter-war years (1918-1939). 1.化学工业的起源 尽管化学品的使用可以追溯到古代文明时代,我们所谓的现代化学工业的发展却是非常近代(才开始的)。可以认为它起源于工业革命其间,大约在1800年,并发展成为为其它工业部门提供化学原料的产业。比如制肥皂所用的碱,棉布生产所用的漂白粉,玻璃制造业所用的硅及Na2CO3. 我们会注意到所有这些都是无机物。有机化学工业的开始是在十九世纪六十年代以William Henry Perkin 发现第一种合成染料—苯胺紫并加以开发利用为标志的。20世纪初,德国花费大量资金用于实用化学方面的重点研究,到1914年,德国的化学工业在世界化学产品市场上占有75%的份额。这要归因于新染料的发现以及硫酸的接触法生产和氨的哈伯生产工艺的发展。而后者需要较大的技术突破使得化学反应第一次可以在非常高的压力条件下进行。这方面所取得的成绩对德国很有帮助。特别是由于1914年第一次世界大仗的爆发,对以氮为基础的化合物的需求飞速增长。这种深刻的改变一直持续到战后(1918-1939)。 date bake to/from: 回溯到 dated: 过时的,陈旧的 stand sb. in good stead: 对。。。很有帮助
完整版化学专业英语
Teaching material for scientific English 一、元素和单质的命名 “元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质”时可用“free element”。因此,单质的英文名称与元素的英文名称是一样的。下面给出的既是元素的名称,同时又是单质的名称。 1主族元素和单质: 2过渡元素和单质 Fe : iron Mn : manganese Cu: copper Zn: zinc Hg: mercury Ag: silver Au: gold 二化合物的命名: 化合物的命名顺序都是根据化学式从左往右读,这与中文读法顺序是相反的。表示原子个数时使用前缀:mono-di -tri-tetra -penta-hexa-hepta-octa-,nona-, deca-,但是在不会引起歧义时,这些前缀都尽可能被省去。 1.化合物正电荷部分的读法: 直呼其名,即读其元素名称。 如CO: carbon monoxide AlO: aluminium oxide 32NO :Di nitrogen tetroxide 42对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous表示低价,-ic表示高价。 如FeO: iron(II) oxide 或ferrous oxide FeO: iron (III) oxide或ferric oxide 32CuO: copper(I) oxide 或cuprous oxide CuO: copper(II) oxide或cupric oxide 22.化合物负电荷部分的读法: 2.1二元化合物: 常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化物,金属氢化物等,命名时需要使用后缀-ide, 如:fluoride,chloride,bromide,iodide,oxide ,sulfide ,nitride, phosphide, carbide,-的
化学化工专业英语(课本内容)
第二章科技英语构词法 词是构成句子的要素,对词意理解的好坏直接关系到翻译的质量。 所谓构词法即词的构成方法,即词在结构上的规律。科技英语构词特点是外来语多(很多来自希腊语和拉丁语);第二个特点是构词方法多,除了非科技英语中常用的三种构词法—转化、派生及合成法外,还普遍采用压缩法、混成法、符号法和字母象形法。 2.1转化法(Conversion) 由一种词类转化成另一种词类,叫转化法。例如: water(n.水)→water(v.浇水) charge(n.电荷) →charge(v.充电) yield(n.产率) →yield(v.生成) dry(a.干的) →dry(v.烘干) slow(a.慢的) →slow(v.减慢) back(ad.在后、向后) →back(v.使后退、倒车) square(n.正方形) →square(a.正方形的) 2.2派生法(Derivation) 通过加前、后缀构成一新词。派生法是化工类科技英语中最常用的构词法。 例如“烷烃”就是用前缀(如拉丁或希腊前缀)表示分子中碳原子数再加上“-ane”作词尾构成的。若将词尾变成“-ane”、“-yne”、“-ol”、“-al”、“-yl”,则分别表示“烯”、“炔”、“醇”、“醛”、“基”、等。依此类推,从而构成千成种化学物质名词。常遇到这样的情况,许多化学化工名词在字典上查不到,全若掌握这种构词法,能过其前、后缀分别代表的意思,合在一起即是该词的意义。下面通过表1举例说明。需要注意的是,表中物质的数目词头除前四个另有名称外,其它均为表上的数目词头。 本书附录为化学化工专业常用词根及前后缀。此外还可参阅《英汉化学化工词汇》(第三版)附录中的“英汉对照有机基名表”、“西文化学名词中常用的数止词头”及“英汉对照有机词尾表”。 据估计,知道一个前缀可帮助人们认识450个英语单词。一名科技工作者至少要知道近50个前缀和30个后缀。这对扩大科技词汇量,增强自由阅读能力,提高翻译质量和加快翻译速度都是大有裨益的。 2.3合成法(Composition) 由两个或更多的词合成一个词,叫合成法。有时需加连字符。 如副词+过去分词well-known 著名的 名词+名词carbon steel 碳钢 rust-resistance 防锈 名词+过去分词computer-oriented 研制计算机的 介词+名词by-product 副产物 动词+副词makeup 化妆品 check-up 检查 形容词+名词atomic weight 原子量 periodic table 周期表 动词+代词+副词pick-me-up 兴奋剂 副词+介词+名词out-of-door 户外 2.4压缩法(Shortening) (1)只取词头字母 这种方法在科技英语中较常用。
化学专业英语
一、元素和单质的命名 “元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质” 时可用“free element”。因此,单质的英文名称与元素的英文名称是一样的。下面给出 的既是元素的名称,同时又是单质的名称。 1主族元素和单质: Fe : iron Mn : manganese Cu: copper Zn: zinc Hg: mercury Ag: silver Au: gold 二化合物的命名: 化合物的命名顺序都是根据化学式从左往右读,这与中文读法顺序是相反的。表示原 子个数时使用前缀:mono- di - tri- tetra - penta- hexa- hepta- octa-, nona-, deca-,但是在不会引起歧义时,这些前缀都尽可能被省去。 1.化合物正电荷部分的读法: 直呼其名,即读其元素名称。 如CO: carbon monoxide Al2O3: aluminium oxide N2O4:Di nitrogen tetroxide 对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的 氧化态,或用后缀-ous表示低价,-ic表示高价。 如FeO: iron(II) oxide 或ferrous oxide Fe2O3: iron (III)oxide或ferric oxide Cu2O: copper(I) oxide 或cuprous oxide CuO: copper(II) oxide或cupric oxide 2.化合物负电荷部分的读法: 2.1二元化合物: 常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化物,金属氢化 物等,命名时需要使用后缀-ide, 如:fluoride, chloride, bromide, iodide, oxide ,sulfide ,nitride, phosphide, carbide,hydride; OH -的名称也是用后缀-ide:hydroxide,
化学专业英语词汇资料
化学专业英语复习题
1.构成原子最重要的的粒子是质子,中子和电子,原子的质量是由核中质子和中子数近似确定的: The most important constitute atomic particles are protons, neutrons and electrons. The total mass of an atom is determined. Very nearly by the total number of protons and neutrons in its nucleus. 2、在元素周期表中,元素的性质是随着原子数周期性变化的,每个周期以活泼的金属元素开始,活泼的非金属结束。从左到右,非金属性逐渐增强,金属性逐渐减弱。 In the periodic table ,the nature of elements changed cyclically with atomic number ,each period begins with a very reactive metal right ,elements show decreasing metallic character and increasing metallic character . 3.当需要在一个官能团化合物的某一个活性选择性的进行反应时,其他活性基团暂时性的被保护起来。很多保护基已经或正在发展用于这个目的。 When a chemical reaction is to be carried out selectively at one reactive site in a multifunctional compound, other reactive sites must be temporarily blocked, many protective groups have been, and are being developed for this purpose. 4、常温下,烷烃和浓硫酸,沸硝酸等不发生化学反应。但在光的引发下易发生氯化反应。该反应是一个自由基键反应,包括链引发,链增长,链终止三个步骤。
应用化学专业英语翻译完整篇
1 Unit5元素周期表 As our picture of the atom becomes more detailed 随着我们对原子的描述越来越详尽,我们发现我们陷入了进退两难之境。有超过100多中元素要处理,我们怎么能记的住所有的信息?有一种方法就是使用元素周期表。这个周期表包含元素的所有信息。它记录了元素中所含的质子数和电子数,它能让我们算出大多数元素的同位素的中子数。它甚至有各个元素原子的电子怎么排列。最神奇的是,周期表是在人们不知道原子中存在质子、中子和电子的情况下发明的。Not long after Dalton presented his model for atom( )在道尔顿提出他的原子模型(原子是是一个不可分割的粒子,其质量决定了它的身份)不久,化学家门开始根据原子的质量将原子列表。在制定像这些元素表时候,他们观察到在元素中的格局分布。例如,人们可以清楚的看到在具体间隔的元素有着相似的性质。在当时知道的大约60种元素中,第二个和第九个表现出相似的性质,第三个和第十个,第四个和第十一个等都具有相似的性质。 In 1869,Dmitri Ivanovich Mendeleev,a Russian chemist, 在1869年,Dmitri Ivanovich Mendeleev ,一个俄罗斯的化学家,发表了他的元素周期表。Mendeleev通过考虑原子重量和元素的某些特性的周期性准备了他的周期表。这些元素的排列顺序先是按原子质量的增加,,一些情况中, Mendeleev把稍微重写的元素放在轻的那个前面.他这样做只是为了同一列中的元素能具有相似的性质.例如,他把碲(原子质量为128)防在碘(原子质量为127)前面因为碲性质上和硫磺和硒相似, 而碘和氯和溴相似. Mendeleev left a number of gaps in his table.Instead of Mendeleev在他的周期表中留下了一些空白。他非但没有将那些空白看成是缺憾,反而大胆的预测还存在着仍未被发现的元素。更进一步,他甚至预测出那些一些缺失元素的性质出来。在接下来的几年里,随着新元素的发现,里面的许多空格都被填满。这些性质也和Mendeleev所预测的极为接近。这巨大创新的预计值导致了Mendeleev的周期表为人们所接受。 It is known that properties of an element depend mainly on the number of electrons in the outermost energy level of the atoms of the element. 我们现在所知道的元素的性质主要取决于元素原子最外层能量能级的电子数。钠原子最外层能量能级(第三层)有一个电子,锂原子最外层能量能级(第二层)有一个电子。钠和锂的化学性质相似。氦原子和氖原子外层能级上是满的,这两种都是惰性气体,也就是他们不容易进行化学反应。很明显,有着相同电子结构(电子分布)的元素的不仅有着相似的化学性质,而且某些结构也表现比其他元素稳定(不那么活泼) In Mendeleev’s table,the elements were arranged by atomic weights for 在Mendeleev的表中,元素大部分是按照原子数来排列的,这个排列揭示了化学性质的周期性。因为电子数决定元素的化学性质,电子数也应该(现在也确实)决定周期表的顺序。在现代的周期表中,元素是根据原子质量来排列的。记住,这个数字表示了在元素的中性原子中的质子数和电子数。现在的周期表是按照原子数的递增排列,Mendeleev的周期表是按照原子质量的递增排列,彼此平行是由于原子量的增加。只有在一些情况下(Mendeleev注释的那样)重量和顺序不符合。因为原子质量是质子和中子质量的加和,故原子量并不完全随原子序数的增加而增加。原子序数低的原子的中子数有可能比原子序数高的原
化学专业英语试卷
2009 —2010学年第一学期 化学与材料学院(系)07级应用化学专业 《专业英语》期末试卷 1.Write the structural formula or Chinese name for each of the following (2% for each answer): (1)barium ion: (2)chlorate ion: (3)potassium ion: (4)carbonic acid: (5)ammonium ion: (6)pyrrole:(吡咯) (7)polystyrene: (聚苯乙烯) (8)p-hydroxybenzoic acid:(对羟基苯甲酸)(9)benzonitrile (苄腈) (10)critical pressure: (临界压力)(11)methanal: (甲醛)(12)buffer solution :(缓冲溶液)(13)alkali burette:(碱式滴定管)(14)extract :(萃取)(15)tetrasulfur dinitride: (S4N2)(16)aldose:(醛醣)(17)sodium dihydrogenphosphate (磷酸二氢钠) (18)zinc oxide: (19)6-ethyl-4-methyldecane: (20)quantitative analysis: (定量分析) 2.Write the English name for each of the following(2% for each answer): (1)IBr: (2)天平(balance)(3)阴离子(anion) (4)H2SO3 (5)滴液漏斗: (dropping funnel)(6)CuNO3: (7)AgF: (8)滴定(n.):(titrate)(9)Ca(MnO4)2: (10)辛醇: (11)十三烷: (12)(CH3CH2)2Hg: (diethylmercury) (13) CH3CHCH CH2 3: (14) CH3CH2CHCOOH CH3: (15)
《化学工程与工艺专业英语》课文翻译Unit 21 Chemical Industry and Environment
Unit 21 Chemical Industry and Environment 化学工业与环境 How can we reduce the amount of waste that is produced? And how we close the loop by redirecting spent materials and products into programs of recycling? All of these questions must be answered through careful research in the coming years as we strive to keep civilization in balance with nature. 我们怎样才能减少产生废物的数量?我们怎样才能使废弃物质和商品纳入循环使用的程序?所有这些问题必须要在未来的几年里通过仔细的研究得到解决,这样我们才能保持文明与自然的平衡。 1.Atmospheric Chemistry Coal-burning power plants, as well as some natural processes, deliver sulfur compounds to the stratosphere, where oxidation produces sulfuric acid particles that reflect away some of the incoming visible solar radiation. In the troposphere, nitrogen oxides produced by the combustion of fossil fuels combine with many organic molecules under the influence of sunlight to produce urban smog. The volatile hydrocarbon isoprene, well known as a building block of synthetic rubber, is also produced naturally in forests. And the chlorofluorocarbons, better known as CFCs, are inert in automobile air conditioners and home refrigerators but come apart under ultraviolet bombardment in the mid-stratosphere with devastating effect on the earth’s stratospheric ozone layer. The globally averaged atmospheric concentration of stratospheric ozone itself is only 3 parts in 10 million, but it has played a crucial protective role in the development of all biological life through its absorption of potentially harmful shout-wavelength solar ultraviolet radiation. 1.大气化学 燃煤发电厂像一些自然过程一样,也会释放硫化合物到大气层中,在那里氧化作用产生硫酸颗粒能反射入射进来的可见太阳辐射。在对流层,化石燃料燃烧所产生的氮氧化物在阳光的影响下与许多有机物分子结合产生都市烟雾。挥发的碳氢化合物异戊二烯,也就是众所周知的合成橡胶的结构单元,可以在森林中天然产生含氯氟烃。我们所熟悉的CFCs,在汽车空调和家用冰箱里是惰性的,但在中平流层内在紫外线的照射下回发生分解从而对地球大气臭氧层造成破坏,全球大气层中臭氧的平均浓度只有3ppm,但它对所有生命体的生长发育都起了关键的保护作用,因为是它吸收了太阳光线中有害的短波紫外辐射。 During the past 20 years, public attention has been focused on ways that mankind has caused changes in the atmosphere: acid rain, stratospheric zone depletion, greenhouse warming, and the increased oxidizing capacity of the atmosphere. We have known for generations that human activity has affected the nearby surroundings, but only gradually have we noticed such effects as acid rain on a regional then on an intercontinental scale. With the problem of ozone depletion and concerns about global warming, we have now truly entered an era of global change, but the underlying scientific facts have not yet been fully established. 在过去的二十年中,公众的注意力集中在人类对大气层的改变:酸雨、平流层臭氧空洞、温室现象,以及大气的氧化能力增强,前几代人已经知道,人类的活动会对邻近的环境造成影响,但意识到像酸雨这样的效应将由局部扩展到洲际范围则是慢慢发现的。随着臭氧空洞问题的出现,考虑到对全球的威胁,我们已真正进入到全球话改变的时代,但是基本的
(完整版)化学专业英语常用词汇
☆常用: ppm: parts per million ppb: parts per billion pH: potential of hydrogen 1. 化合物的命名:规则:金属(或某些非金属)元素+阴离子名称 (1)MgCl2 magnesium [m?ɡ’ni:zj ?m] chloride (2)NaNO2 sodium nitrite [‘naitrait] (3)KNO3 potassium[p ?’t?si ?m] nitrate [‘naitreit] (4)硝酸 nitric acid (5)NaHCO3 sodium hydrogen carbonate 练习: ? FeBr2 ? (NH4)2SO4 ? NH4H2PO4
?KMnO4 ?亚硫酸 ?sulfurous acid ?H2S ?NO 2 有机物命名 ?Hydrocarbon ?{Aliphatic hydrocarbon; Aromatic Hydrocarbon} ?Aliphatic hydrocarbon (脂肪烃) ?{Alkane (烷); Alkene(烯); Alkyne(炔)} ?Alcohol 醇 ?Aldehyde 醛 ?Ketone [‘ki:t?un] 酮 ?Carboxylic acid 羧酸 ?Aromatic hydrocarbon(芳香烃) ?{benzene (苯) hydroxybenzene(酚) quinone(醌) 无机物中关于数字的写法 mono-, di-, tri-, tetra-, penta- hexa-, hepta-, octa-, nona-, deca- 一,二,三,四,五,六,七,八,九,十 有机物中关于数字的写法 meth-, eth-, prop-, but-, pent-, hex-, 甲乙丙丁戊已 hept-, oct-, non-, dec-, cyclo-, poly- 庚辛壬葵环聚 练习 ?甲烷乙炔 ?丙酮丁醇 ?戊烷己烯 ?庚醛辛烷 ?2-甲基壬酸 3,5-二乙基癸醇
应用化学专业英语第二版万有志主编版课后答案和课文翻译
Unit 1 The RootsofChemistry I.Comprehension. 1。C 2. B3.D 4. C 5. B II。Make asentence out of each item by rearranging the wordsin brackets. 1.Thepurification of anorganic compoundis usually a matter of considerabledifficulty, and itis necessary to employ various methods for thispurpose。 2.Science is an ever-increasing body ofaccumulated and systematized knowledge and isalsoan activity bywhic hknowledge isgenerated。 3.Life,after all, is only chemistry,in fact, a small example of c hemistry observed onasingle mundane planet。 4.Peopleare made of molecules; someof themolecules in p eople are rather simple whereas othersarehighly complex。 5.Chemistry isever presentin ourlives from birth todeathbecause without chemistrythere isneither life nor death. 6.Mathematics appears to be almost as humankindand al so permeatesall aspects of human life, although manyof us are notfully awareofthis. III。Translation. 1.(a)chemicalprocess (b) natural science(c)the techni que of distillation 2.Itis theatoms that makeupiron, water,oxygen and the like/andso on/andsoforth/and otherwise. 3.Chemistry hasa very long history, infact,human a ctivity in chemistrygoes back to prerecorded times/predating recorded times. 4.According to/Fromthe evaporation ofwater,people know /realized that liquidscan turn/be/changeinto gases undercertain conditions/circumstance/environment。 5.Youmustknow the propertiesofthe materialbefore y ou use it. IV.Translation 化学是三种基础自然科学之一,另外两种是物理和生物.自从宇宙大爆炸以来,化学过程持续进行,甚至地球上生命的出现可能也是化学过程的结果。人们也许认为生命是三步进化的最终结果,第一步非常快,其余两步相当慢.这三步
化学专业英语修订版翻译
. 01 THE ELEMENTS AND THE PERIODIC TABLE 01 元素和元素周期表 The number of protons in the nucleus of an atom is referred to as the atomic number, or proton number, Z. The number of electrons in an electrically neutral atom is also equal to the atomic number, Z. The total mass of an atom is determined very nearly by the total number of protons and neutrons in its nucleus. This total is called the mass number, A. The number of neutrons in an atom, the neutron number, is given by the quantity A-Z. 在一个原子核中的质子数量被称为原子序数,或质子数,Z。在一个电中性原子中的电子数量也等于原子序数,Z。一个原子的总质量被测定是非常接近于原子核中质子和中子的总数。这个总数被称为质量数,A。在一个原子中的中子数量等于A –Z的数量。 The term element refers to, a pure substance with atoms all of a single kind. To the chemist the kind of atom is specified by its atomic number, since this is the property that determines its chemical behavior. At present all the atoms from Z = 1 to Z = 107 are known; there are 107 chemical elements. Each chemical element has been given a name and a distinctive symbol. For most elements the symbol is simply the abbreviated form of the English name consisting of one or two letters, for example: 这个术语(指chemical element)也可以指由相同质子数的原子组成的纯化学物质。对化学家来说,这类原子通过原子数来说明,因为它的性质是决定其化学行为。目前,从Z = 1 到Z = 107的所有原子是知道的;有107种化学元素。每一种化学元素起了一个名字和独特的象征。对于大多数元素都仅仅是一个象征的英文名称缩写形式,由一个或两个字母组成,例如:oxygen==O nitrogen == N neon==Ne magnesium == Mg 氧= =O 氮= = N氖= = Ne 镁= =Mg .. . Some elements,which have been known for a long time,have symbols based on their Latin names, for example: 很久以来就已经知道一些元素,根据他们的拉丁名字符号命名,例如: iron==Fe(ferrum) copper==Cu(cuprum) lead==Pb(plumbum) 铁= =铁(铁) 铜= =铜(铜) 铅= =铅(铅)
